Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
The
density of liquid mercury is 13.5 g/cm3. What mass of mercury is required to fill a 2.00 L
container? a. | 6.75
´ 10-3
g | b. | 1.48
´ 10-2
g | c. | 6.75
g | d. | 1.48
´ 102
g | e. | 2.70
´ 104
g | | |
|
|
|
2.
|
The
boiling point of liquid helium is -269°C. What is this temperature in kelvin? a. | 1.01
K | b. | 4
K | c. | 77
K | d. | 269
K | e. | 542
K | | |
|
|
|
3.
|
What
is a correct method for converting Celsius to Kelvin? a. | t(K) = 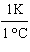 (t°C - 273.15°C) (t°C - 273.15°C) | b. | t(K) = 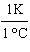 (t°C - 273.15°C) (t°C - 273.15°C) | c. | t(K) = 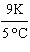 (t°C - 273.15°C) (t°C - 273.15°C) | d. | t(K) = 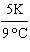 (t°C - 273.15°C) (t°C - 273.15°C) | e. | none of the
above | | |
|
|
|
4.
|
A
common wavelength of light emitted from a red laser pointer is 6.50 ´ 102
nm. What is the wavelength in meters? a. | 6.50 ´ 10-9 m | b. | 6.50
´ 10-7
m | c. | 6.50
´ 10-5
m | d. | 6.50
´ 10-3
m | e. | 6.50
´ 100
m | | |
|
|
|
5.
|
If
the fuel efficiency of an automobile is 22 miles per gallon, what is its fuel efficiency in
kilometers per liter? (1 km = 0.621 mile, 1.000 L = 1.057 quarts, 4 quarts = 1
gallon) a. | 3.2
km/L | b. | 3.6
km/L | c. | 9.4
km/L | d. | 32
km/L | e. | 52
km/L | | |
|
|
|
6.
|
A
gallon (3.78 L) of latex paint can cover 385 ft2 of the surface of a wall. What is the
average thickness of one coat of paint (in micrometers)? a. | 0.781
mm | b. | 1.42 mm | c. | 14.2
mm | d. | 36.7 mm | e. | 106
mm | | |
|
|
|
7.
|
Silver iodide (AgCl) is relatively insoluble in water. At 25 _C, only 214 mg
(micrograms) will dissolve in 1.0 liter of water. How many liters of water are needed to dissolve 5.0
g of silver iodide? a. | 4.3 ´ 100 L | b. | 2.1 ´ 102
L | c. | 4.3 ´ 102
L | d. | 1.1 ´ 103
L | e. | 2.3 ´ 104
L | | |
|
|
|
8.
|
Express 5.00 ´ 10-2 m in standard notation using the correct number of
significant figures. a. | 0.05 cm | b. | 50
mm | c. | 50.0
mm | d. | 500
m | e. | 500.
m | | |
|
|
|
9.
|
What
is the correct answer to the following expression: 3.33 ´ 10-5 + 8.13 ´
10-7? a. | 3 x
10-5 | b. | 3.4 ´ 10-5 | c. | 3.41
´
10-5 | d. | 3.411 ´ 10-5 | e. | 3.4113
´
10-5 | | |
|
|
|
10.
|
Express 0.05620 in exponential notation. a. | 5.6 ´
10-2 | b. | 5.62 ´ 10-2 | c. | 5.620
´
10-2 | d. | 5.6 ´ 102 | e. | 5.62
´
102 | | |
|
Numeric Response
|
|
|
11.
|
Enter
your score on the Module Two multiple choice, so that your improvement may be measured and your M-2
test score may be adjusted:
|