Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
Rank
the subatomic particles from lowest to highest mass? a. | electrons <
neutrons < protons | b. | electrons < protons < neutrons | c. | electrons <
protons = neutrons | d. | neutrons < electrons < protons | e. | electrons =
protons < neutrons | | |
|
|
|
2.
|
Which
two of the ions below have the same number of electrons?
a. | | b. | | c. | | d. | | e. | none of the above | | |
|
|
|
3.
|
How
many electrons are in 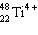 ? ?
|
|
|
4.
|
What
alkaline earth metal is in the fourth period?
|
|
|
5.
|
Which
of the following is associated with the value of the ª quantum number? a. | wave
function | b. | principle quantum number | c. | orbital
orientation | d. | orbital shape | e. | number of
electrons | | |
|
|
|
6.
|
Which
of the following diagrams represent a p-orbital?
a. | (I)
only | b. | (II)
only | c. | (III)
only | d. | (IV)
only | e. | (I) and
(II) | | |
|
|
|
7.
|
What
is the electron configuration for Cu+? a. | [Ar] | b. | [Ar]3d8 | c. | [Ar]4s23d8 | d. | [Ar]3d10 | e. | [Ar]4s13d9 | | |
|
|
|
8.
|
What
is the electron configuration for Pb2+? a. | [Xe]5d106s2 | b. | [Xe]4f145d106s2 | c. | [Xe]6s2 | d. | [Xe]4f145d10 | e. | [Xe]4f146p2 | | |
|
|
|
9.
|
Hund's rule states that the most stable arrangement of electrons (for a ground state
electron configuration) a. | has two electrons per orbital, each with opposing
spins. | b. | has two electrons per orbital, each with identical
spins. | c. | only occurs for s and p-block
elements. | d. | has a filled valence shell of
electrons. | e. | has the maximum number of unpaired electrons, all with the same
spin. | | |
|
|
|
10.
|
What
element has the following electron configuration?
|
|
|
11.
|
What
element has the following electron configuration?
|
|
|
12.
|
What
-1 ion the following electron configuration?
|
|
|
13.
|
What
is a possible set of quantum numbers for the unpaired electron in the orbital box diagram
below?
a. | n = 3,
ª = 1,
mª = -1, ms =
+1/2 | b. | n = 4,
ª = 1,
mª = -1, ms =
+1/2 | c. | n = 3
ª = 2,
mª = -2, ms =
+1/2 | d. | n = 3,
ª = 2,
mª = -1, ms =
-1/2 | e. | n = 4,
ª = 2,
mª = 0, ms = +1/2 | | |
|
|
|
14.
|
Which
group of the periodic table elements forms only +1 ions? a. | group
1A | b. | group
2A | c. | group
7B | d. | group
7A | e. | group
8A | | |
|
|
|
15.
|
Place
the following ions in order from smallest to largest radii: N3-, F-,
Cl-, Mg2+, and Li+. a. | Mg2+
< Li+ < Cl- < N3- <
F- | b. | Li+ < Mg2+ < F- <
N3- < Cl- | c. | Li+ < Mg2+ < N3- <
F- < Cl- | d. | F- < N3- < Li+ <
Mg2+ < Cl- | e. | F- < N3- <Mg2+ <
Li+ < Cl- | | |
|
Numeric Response
|
|
|
16.
|
Enter
the score on your Module Three multiple choice test below
|