Chapter 3 Section 3.5
Guideline #1 Tasks #1 & #2 Study Guide
††††††††††† 
1.
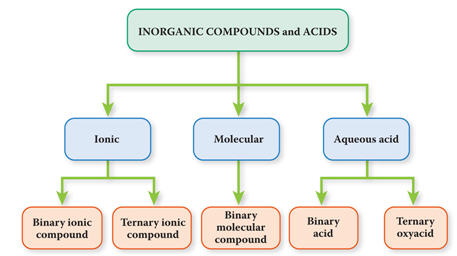
Inorganic Compounds are subdivided into three Categories:
a. Ionic* :†††††††† Metal + Nonmetal †
b. Molecular:
†Nonmetal-Nonmetal
c. Acids:††††††††††† Hydrogen + Nonmetal in aqueous
solution
*(also called Salts, Minerals, and Body
Electrolytes)
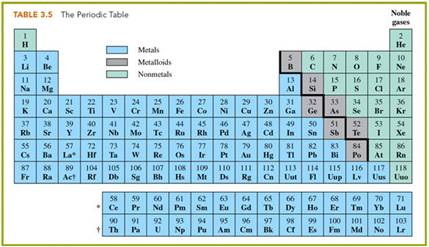
††††† 2. Look at the periodic Chart to
differentiate Metals from Nonmetals
†††††††††††††††††† 
†††† Task #11 & #12: Classify Inorganic
Compound
††††††††††† By
the Element Written 1st in the Formula
††††††††††††††††††††††††††††††† (see #1 above)
Task #11: Inorganic Compound Names
Task #12: Inorganic Compound Formulas
--------------------------------------------------------------------------------
Task #1: BINARY (IONIC) COMPOUND Names
†††††††††††† 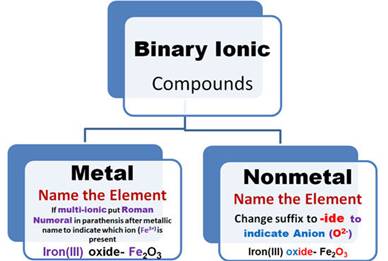
†To be an Ionic Compound:
a.† the element written first
in either the name or the formula is a
metallic ion (Cation).
††††††††††††††††††
b. The element written second
is a nonmetallic ion (Anion).
††††††††††††††††††
c.† Salts are metallic and nonmetallic ionic compounds
†††††† †
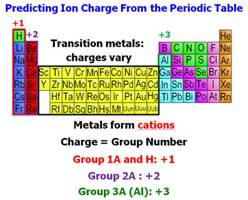
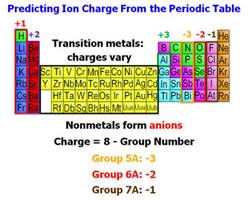
†††† Predict the Charge on the Cation and
Anion (Use the Periodic Chart).
†††††††††††
††††††††††††††††††† †††††Cation†††††††††††††††††††††††††††††††††††††††††††††
Anion
††††††  †††††
†††††
†††† d. There are no molecules of salts-just macro ionic lattices.
†††† e. Name the metallic element.
†††††††††††††††††
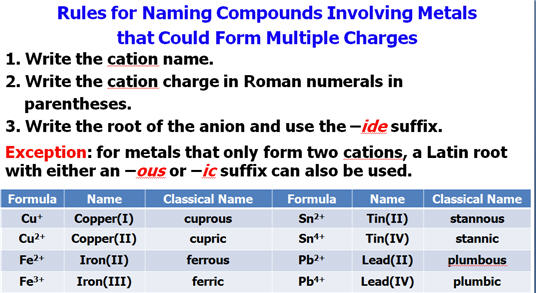
†††† †f.†† If the metallic
element has more than one ionic state,
††††††††††† write a ROMAN
NUMERAL after
the elementís name in parenthesis†
†††††††††† †to indicate which charge
state the metallic element is using to form the compound.
g.†† Name the nonmetallic element
name from the formula:
††††† 1. Drop the suffix
off the nonmetalís name and add -ide which indicates the
salt is binary
(exceptions: cyanide &
hydroxide which are polyatomic ions).
.
†Examples:
†††††††††† NaCl
Sodium Chloride (table salt)
††††††††† Al2O3
Aluminum oxide
††††††††† FeS Iron( II) sulfide† (Latin
Name Ferrous Sulfide)
††††††††† Fe2O3
Iron(III) oxide*†
(Latin Name Ferric oxide)
†††††††††††††††††††††††††††††††††††††††† *(also called rust as explained in Guideline 4)
To complete Project
#5 Task#1, you will write the names of 10 Binary Ionic Compounds from the
formula.( Do not forget to put the Roman numeral if the element has
more than one ionic charge possible [Transitional metals])
Task #1 Link:
http://www.fscj.me/nomenclature/BinarySalts/Project5BinaryIonicNames.html
-------------------------------------------------------------------------------------------------------------------------------------------
Task #2: Binary
(ionic) Compound Formulas
BINARY (IONIC)
COMPOUND Formulas
To write the formula from the name of the salt
†Use the following procedure:
(a) Write the symbols (or formulas for
radicals) of the ions represented
††††††
For Example:
†††††††††††††††††††††† ††††††††††††††††††††††††††† Calcium nitride
†† Ca N
 †
† 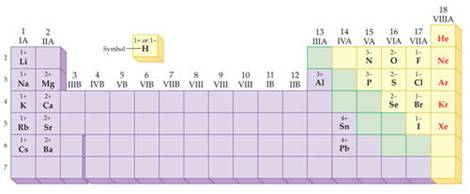
(c) Use the periodic chart to write the ion charge of
each element (or polyatomic ion) as ††††††††††††††††††††††† Calcium nitride
†††††† Ca+2 N-3
(d ) Find the L.C.M. (Least common multiple) of the positive and negative charge.
The LCM is the smallest number that both charges will decide into evenly. The LCM is the total electrons transferred. Therefore, it represents the total positive charge created by the metallic ions and the total negative charge created by the nonmetallic ions. This may be proved by drawing the dot structure of the compound showing all electrons transferred.
The LCM of +2
and -3 is 6,
†therefore
6 e-1 are transferred creating a total positive
charge of +6,
and the total
negative charge of -6
†††††††††††††† +6 --> 6e-1-->
-6
††††††††††††††† Ca+2 †††† N-3
(d (d) Divide the LCM by the positive charge,
this dividend will represent the subscript behind the metallic ion in the
formula.
+6 divided by +2 = 3; therefore half of the formula is:
††††††††††††††††††††††† Ca3Nx
(e) Divide the LCM by the negative charge, this dividend will represent the number of nonmetallic ions in the formula.
-6 divided by -3 = 2; therefore
the other half of the formula is:
††††††††††††††††††††††††††††††††††††††††††††††††††††††
Ca3N2
--------------------------------------------------------------------------------------------------------------------------------------------------
†††††† ††††††
2nd
Example: Potassium phosphide
††††††††††††††††††††††††††††††††††††††††††††† Write Charges:
†† †††††††K+1 P -3
†††††††††††††††††††††††††††††††††††††††††††††††
LCM: 3
†††††††††††††††††††††††††††††††††††††††††††††††††††††††††††
Balance the chemical formula:
†††††††††††††††††††††††††† †K3P
3rd
†Example:
††††††††††††††††††† 
To complete Project
#5 Task#2, you will write the formulas of 10 Binary Ionic Compounds from the
name. (Do not forget the
Roman numeral if the element has more than one ionic charge possible [most Transitional
metals])
Task #2 Link:
http://www.fscj.me/nomenclature/BinaryIonicFormula/Project5BinaryIonicFormula.html