Chapter 3 Section 3.5 Guideline #3 Tasks #7 & #8 Study Guide
†††††††††††††††††††††††††† Ternary Ionic
Compounds
††††††††††
††††††††
1.
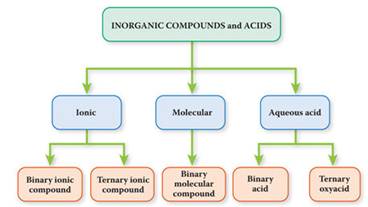
Inorganic Compounds are subdivided into three Categories:
a. Ionic* : †Metallic (Cations) + Nonmetallic (anions) (ion smallest unit)
b. Molecular:
†Nonmetal-Nonmetal
(molecule smallest unit)
c. Acids
(aq): †Hydrogen ions (Hydronium
ions) +
Nonmetallic ions (Cations)
†††††††††††††††††††††††††† (in aqueous
solution)
*(also called Salts, Minerals, and Body
Electrolytes)
††††† 2. Ternary ionic means that there are
three or more elements (ternary)
†††††††††† in the formula and the
compound is made up of two ions (ionic):
††††††††††† a.† at least one of the ions (either the CATION
or ANION or both)
††††††††††††††† †is/are polyatomic ion(s) (See Polyatomic
ion study guide)
†††††††††††
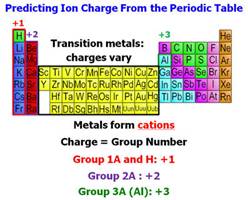
b. †Positive Charged Metallic ion
(CATION): Metallic Cation is a
††††††††††††††† †positively charged metallic atom as explained
in Guideline
1 or
†††††††††††
c.† Positive
Charged Polyatomic CATION is a group of two or more
†††††††††††††††† nonmetal atoms covalently
bonded but the group has an overall
†††††††††††††††† charge positive charge.
††††††††††††††††††††††††† †††Ammonium
NH41+ and Hydronium H3O1+
†††††††††††††††† are the two Polyatomic Cations mostly used from Polyatomic ion
†††††††††††††††† list.
††††††††††††
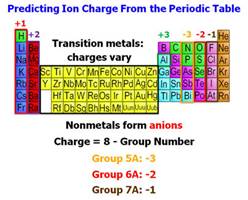
d. Negative Charged Nonmetallic Ion (ANION) is group of two or
†††††††††††††††† more nonmetals atoms
covalently bonded but the group has
†††††††††††††††† an overall negative
charge. The majority of Polyatomic ions are
†††††††††††††††† Anions composed a
nonmetal or transitional metal bonded to
†††††††††††††††† oxygen. All nonmetals
(except fluorine), some transitional
†††††††††††††††† metals, and many
metalloids bond with oxygen to make these
††††††††††††††† †Anions. These ions are often called oxyanions. The Master
list
††††††††††††††† †has over 130 polyatomic Anions.
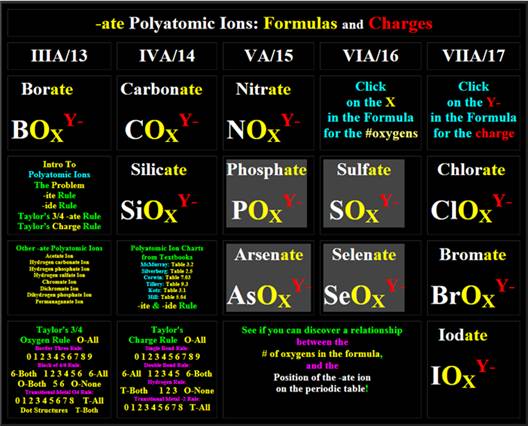
3. Look at the list of
polyatomic ions below we will use when we
††††††††† write the Names or Formulas of
make Ternary Ionic Compounds:†††††††  ††
††
†††
††† †-ate
Polyatomic ions with four oxygen atoms
†††† -ate Polyatomic ions with three oxygen atoms
†††† Polyatomic ions which also contain hydrogen
†††† -ide Polyatomic ions
(and also Cyanate which is different)
†††† Positive Charged (Cation)
Polyatomic Ions
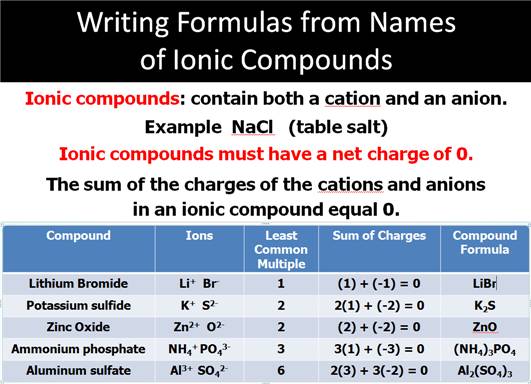
4. The total charge on the
Ternary ionic compound must add up to zero like the Binary Ionic Compounds (Guideline
#1). Use the Least Common Multiple technique explained in Guideline #1 to
balance the formula. To indicate more than one polyatomic ion group enclose the
group in parenthesis and apply the subscript to the group.
5.
For example, write the formula and charges of the ions, then balance using the
LCM:
†††††††††††††††† Cation†††††††††††††††††††††††††††††††††† Anion
† ††
†† 
Magnesium phosphate††††† Ammonium Carbonate†† ††Calcium hypochorite†
†† Mg2+† †††††††PO43-††††††††††††††††† NH41+††††† CO32-†††††††††† †Ca2+††††† ClO1-
†††† The LCM is six †††††††††††††††††††The LCM is 2††††††††††††††††††† The LCM is two
††††† †Mg3(PO4)2†††††††††††††††††††††† (NH4)2CO3†††††††††† ††††††††††††††Ca(ClO)2
††
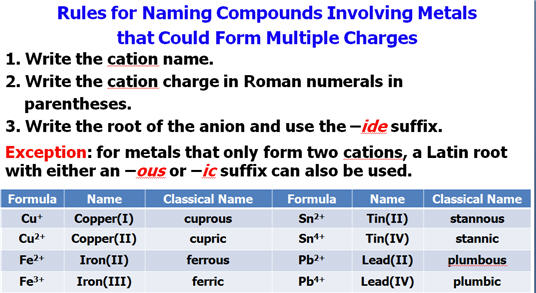
†Do Not Forget if the metallic
element has more than one ionic state,
††††††††††† write a ROMAN
NUMERAL after
the elementís name in parenthesis†
††††††††††† to indicate which charge state the metallic element is using
to form the compound.
††††††† 
†††††††††††† †
. 
To complete Project
#5 Task#7, you will write the names of 10 Ternary Ionic Compounds from the
formula.( Do not forget to put the Roman numeral if the element has
more than one ionic charge possible [Transitional metals])
Task #7: Ternary (IONIC) COMPOUND Names
†
To complete Project
#5 Task#8, you will write the formulas of 10 Ternary Ionic Compounds from the
name. (Do not forget the
Roman numeral if the element has more than one ionic charge possible [most Transitional
metals])
Task #8: Ternary (IONIC) COMPOUND Formulas
Sample Ternary Ionic Compound Names & Formulas
Practice
Using
a periodic chart write the names or formulas of the following compounds
depending on whether the formula or name is given:
1.†† Na2CO3†††††† __Sodium carbonate_______
2.†† K2SO4†††††††††† __Potassium sulfate_______
3.†† (NH4)3PO4† ††__Ammonium phosphate___
4.†† Ca(ClO3)2†††† __Calcium chlorate________
5.†† CuNO3†††††† †††††__Copper I Nitrate_________
6.†† Aluminum Hydroxide††††††††† __Al(OH)3_____
7.†† Ammonium carbonate††††††††† __(NH4)2CO3___
†8.†† Sodium Hypochlorite††††††† ††††___NaClO_____
9.†† Magnesium Nitrate†††††††††††††† ___Mg(NO3)2____
10.† Iron III sulfite†††††††††††††††††††††† ___Fe2(SO3)3___
Discover
Polyatomic Ion Formula and Charges from Interactive Web Page:
Click image below: