Chapter 3 Section 3.5 Guideline #5
Tasks #9 & #10
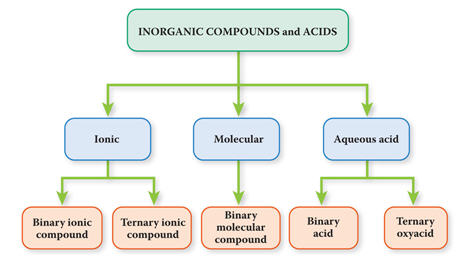
Binary -Ternary Acids Names and
Formulas
•
Fifth guideline (Not in the Book)
Compounds which have Hydrogen written first in
the
formula and are in aqueous solutions (aq)
are known as Binary and Ternary
Acids
HClO4 (aq) – Perchloric Acid
HClO3 (aq) – Chloric Acid
HClO2 (aq) – Chlorous Acid
HClO (aq) – Hypochlorous Acid
HCl (aq) – Hydrochloric Acid
Acids are
classified as Strong Acids and Weak Acids (Chapter 10)

Strong Acids Weak Acids
Strong acids ionize 100% in a water solution, while
Weak Acids ionize less than 5% in a water solution.
A brief tutorial for names and formulas of acids follows:
If hydrogen is
written first in a chemical formula, there are two ways to
name the compound.
1. As a pure molecular compound or
2. As an aqueous acid:
Step #1: If the compound is a pure molecular compound then you name it just as if it were an ionic compound:
HCl
hydrogen chloride H3PO4
hydrogen phosphate
HClO
hydrogen hypochlorite H2SO4
hydrogen sulfate
HClO2
hydrogen chlorite
H2SO3 hydrogen
sulfite
HClO3
hydrogen chlorate
HBr
hydrogen bromide
HClO4
hydrogen Perchlorate
HF hydrogen fluoride
H2CO3
hydrogen carbonate HI hydrogen iodide
HC2H3O2
hydrogen acetate
H2C2O4 hydrogen oxalate
Writing hydrogen
first in a chemical formula indicates that when you dissolve the compound
in water, a water molecule has the ability to pull the hydrogen off (from strong electronegative elements like
oxygen) the molecule HXO3 and creating hydronium ions, H3O1+ and a negative ion XO31- (cation).
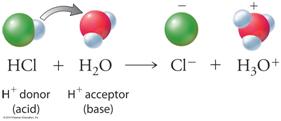
The way you indicate this ionic solution is to write the formula followed by (aq) meaning a water solution: HXO3 (aq) .
Step
#2 is to drop the first word hydrogen and
add a second word acid:
HCl hydrogen chloride acid (aq)
HClO hydrogen hypochlorite acid (aq)
HClO2
hydrogen chlorite acid (aq)
HClO3
hydrogen chlorate acid (aq)
HClO4
hydrogen perchlorate acid (aq)
H3PO4
hydrogen phosphate acid (aq)
H2CO3
hydrogen carbonate acid (aq)
H2SO4
hydrogen sulfate acid (aq)
H2SO3 hydrogen sulfite acid (aq)
HC2H3O2
hydrogen acetate acid (aq)
H2C2O4 hydrogen oxalate acid (aq)
HBr
hydrogen bromide acid (aq)
HF hydrogen fluoride acid (aq)
HI
hydrogen Iodide acid (aq)
Step #3 is to drop the suffix from the ANION and make the following substitution with another suffix:
Change the -ate to -ic
Change the -ite
to -ous
but instead of coming
up with a third suffix for -ide ,
they reused the -ic for -ide and added
a prefix hydro-
(Do not get this confused
with the prefix hypo- which means
'under'.)
HCl hydrochloric acid (aq)
HClO hypochlorous
acid (aq)
HClO2 chlorous acid (aq)
HClO3
chloric acid (aq)
HClO4
perchloric acid (aq)
H3PO4
phosphoric acid (aq) (Put the -or- syllable back in the name)
H2CO3
carbonic acid (aq)
H2SO4
sulfuric acid (aq) (Put the -ur- syllable back in
the name)
H2SO3
sulfurous acid (aq) (Put the -ur- syllable back in
the name)
HC2H3O2
acetic acid (aq) (Notice the three hydrogens
written after carbon are
NOT ionizable and not written first in the
formula)
H2C2O4 oxalic acid (aq)
HBr
hydrobromic acid (aq)
HF hydrofluoric acid (aq)
HI hydroiodic acid (aq)
Chapter 3 Section 3.5 Guideline #5
Tasks #9 & #10
Task
#9 Names of Binary-Ternary Acids
http://www.fscj.me/Nomenclature/Acids/Project5AcidNames.html
Task
#10 Formulas of Binary-Ternary Acids
http://www.fscj.me/Nomenclature/AcidFormulas/Project5AcidFormula.html
