REDOX versus Non-REDOX Reactions
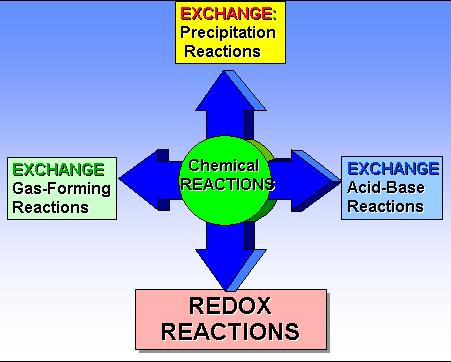

on REDOX Reactions have no changes in ionic charges during chemical change. No covalent bonds are made or broken during the reactions. The Precipitation Reactions form Insoluble Salts(s) in the product(s).
In Acid-Base Neutralization Reactions a covalent bond is between the Hydrogen Ion and the Hydroxide Ion. Sometimes the other product is an insoluble salt. When an acid is added to an insoluble base, the base disolves while forming the water molecules.
In Gas Forming Reactions, a product like H2CO3 or NH4OH is one of the product which instantly decomposes into a gas and water.
xidation-Reduction Reactions occur when covalent bonds are broken and new covalent bonds are formed. In the process, one of the reactants gains electrons and one of the reactants loses electrons:
Losing Electrons is oxidation, while Gaining electrons is Reduction.
Look at the possibilities of element X breaking apart from element Y:
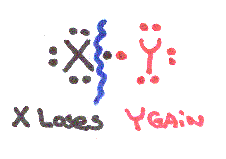
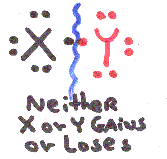
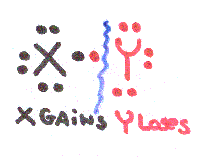
Most Covalent Bonds break unevenly, if X & Y have different electronegativities so that one atom gains electrons and the other loses.