CHM
1020 Path 4 Chapter 9 Study Pack Part III
Chapter 9
Part III: Chemical Equations & Stoichiometry (Chapter 9)
F1.Mass-Mass Stoichiometric Problems- Answers
F2.Excess/Limiting Reagent Problems-
Answers
F3.Per Cent Yield Problems/Impure Reagents-Answers
Chapter
9 Part F Chapter
9

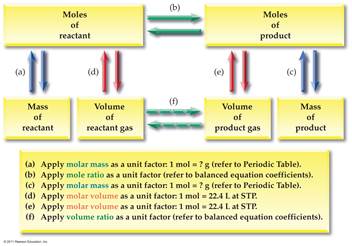
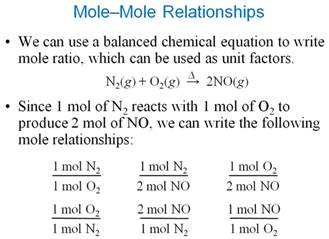
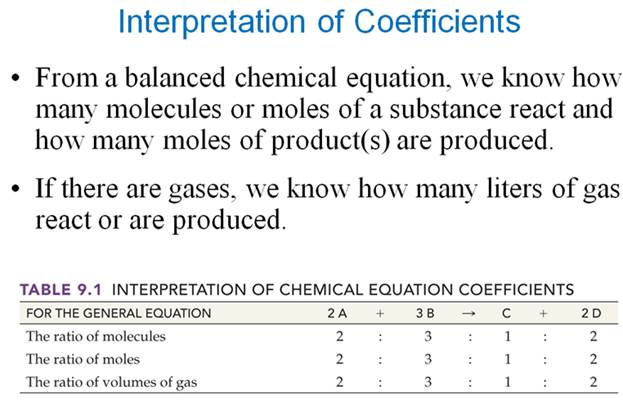
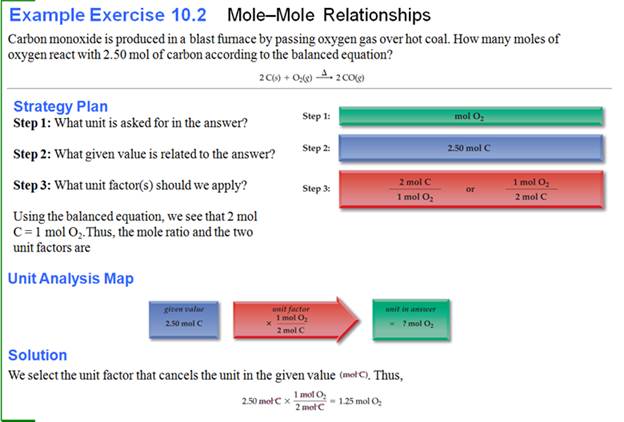
Part F Mole-Mole
Stoichiometry
Homework #1: Tungsten occurs in the important mineral sheelite (Calcium tungstate), which is converted to
tungstic acid. Tungsten is then
extracted from tungstic acid by the following (unbalanced) reaction:
H2 +
H2WO4 à W
+ H2O
How moles of
hydrogen is needed to prepare 6 moles of elemental
tungsten?
Homework #2: Phosphoric acid can be made by the following (unbalanced) reacti
H2O + P4O10 à
H3PO4
How many moles of
Phosphoric acid can be prepared from the combination of 5 moles of Tetraphosphorus decoxide with
excess water?
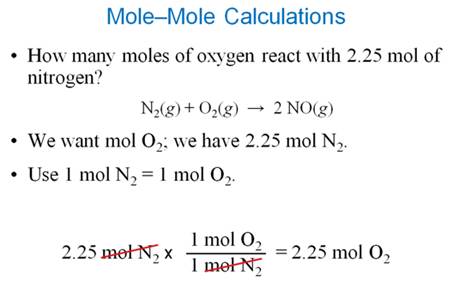
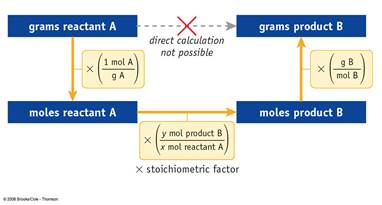
Part F2 Mass-Mass
Stoichiometry
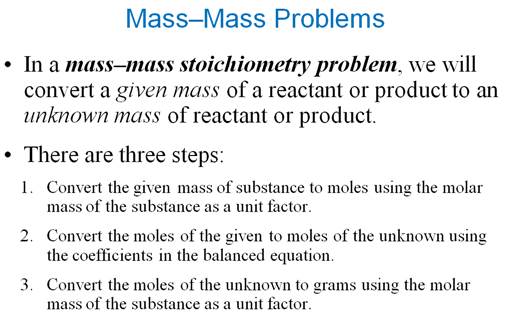
Use this concept map for Part F2 Mass-Mass Problems:
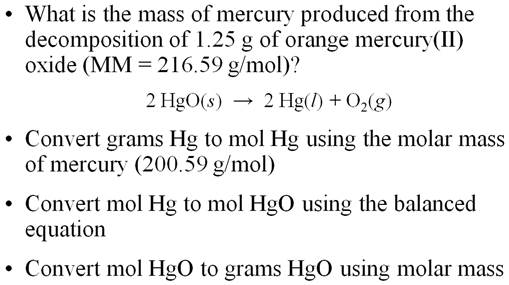
The Solution to: __?____gHg = 1.25g 1.25 gHgO
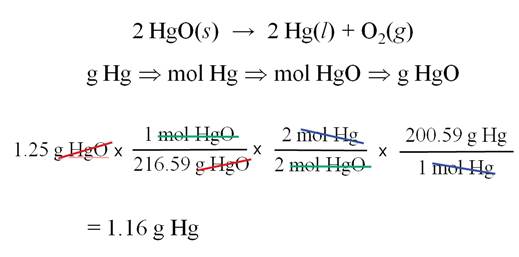

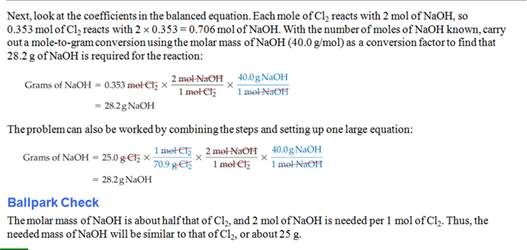
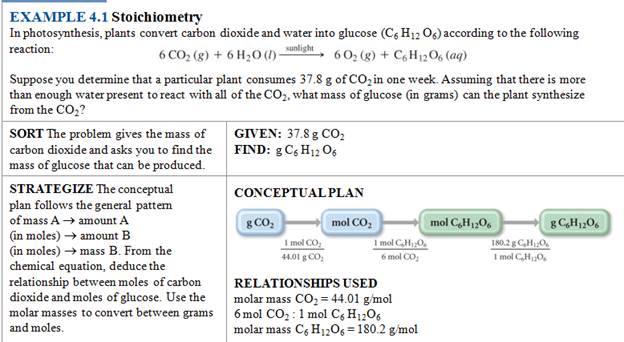

Homework
#3: Toluene and nitric acid
are used in the production of trinitrotoluene (TNT), an explosive:
C7H8 +
HNO3 à C7H5N3O6 +
H2O (Unbalanced)
Calculate the mass
of TNT that can be made from 192 g of C7H8 (toluene).
Homework
#4: What mass of carbon dioxide is produced from
the combustion of 176 grams of propane gas , C3H8 , in excess oxygen
gas, O2. Water is the only other product.
Part F3
Excess-Limiting Reagent Problem
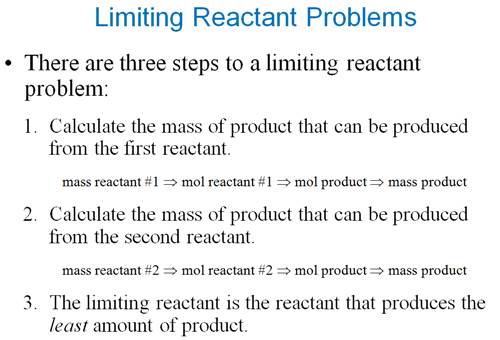
Sample Limiting Reagent Problem
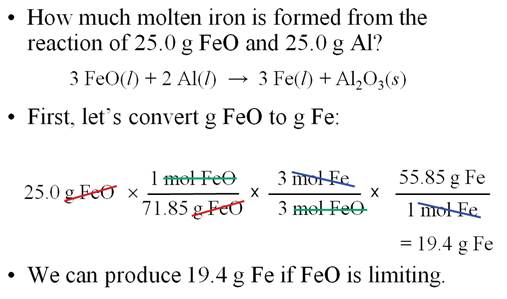
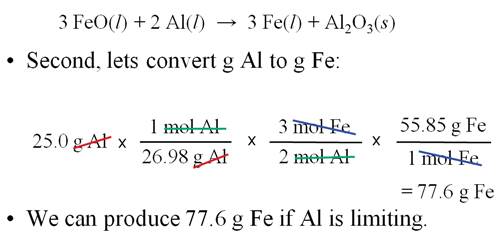

Some books teach you to determine
which reagent is the limit first, then do the standard gram-gram problem. The
following is an example of this method.
Either works, but I prefer the method above.
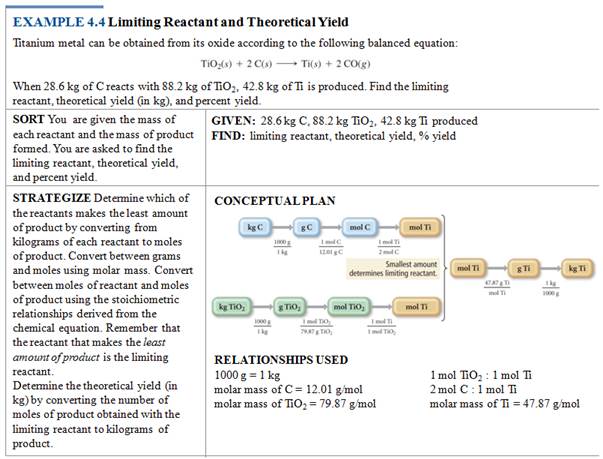
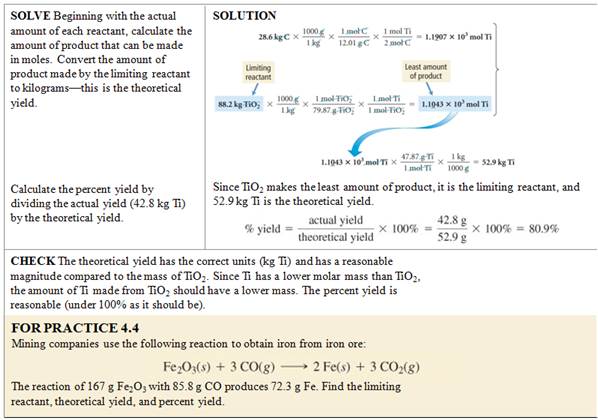
Homework #5: How
many grams of Calcium phosphate can be made according to the reaction
(unbalanced):
CaCl2 + K3PO4 ----> Ca3(PO4)2 + KCl
by
mixing a solution of 5.00 grams of CaCl2 with another
containing 8.00 grams of Potassium phosphate?
Part F3 Impure Reagents/Percent
Yield Problem
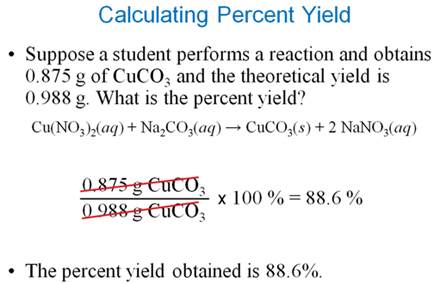
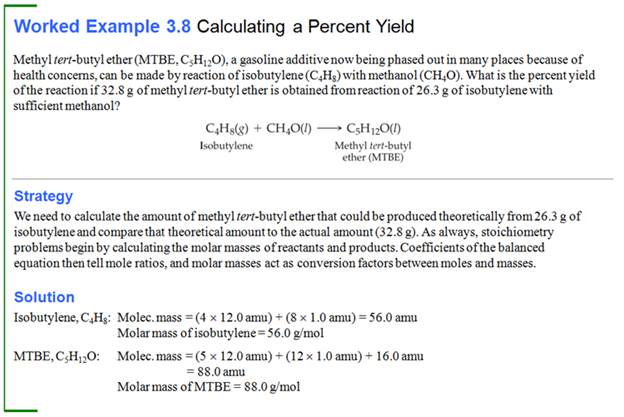
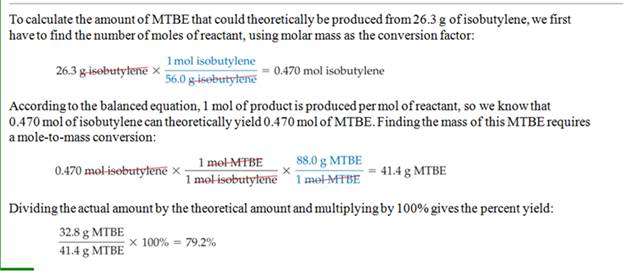
Homework #6: A laboratory manual calls for 13.0
grams of butanol reactant in excess sodium bromide and sulfuric acid as
reactants in this reaction:
C4H9OH + NaBr + H2SO4 ------> C4H9Br + NaHSO4 + H2O
A
student following these directions obtains 16.8 grams of butyl bromide (C4H9Br). What is the theoretical yield and the
percent yield of this reaction?