CHM 1020 Path 4 Chapter 9 Study Packet Part II
Chapter 9 Part II: Chemical
Equations
C. Molecular Mass
Calculation- Answers
D._Mole Calculations I-Sections 8.2 old sample pretest: Answers bcd
D1.Mole Calculation II-Section 8.4 old sample pretest: Answers bcd
E._Percentage Composition Calculation-Section 8.7 old sample pretest Answers bcd
E1.Empirical Formula Calc. from Lab Data-Section 8.8 old preterst Answers bcd
E2.Empirical Formula
Calc. from % Comp-Section 8.8
old pretest Answers
bcd
______
Total
Chapter 9-Part C: Molecular Mass Calculation
The atomic mass of any substance expressed in grams is the molar mass
(MM) of that substance.
The
atomic mass of carbon is 12.01 amu per atom.
Therefore,
the molar mass of carbon is 12.01 g/mol .
Since
nitrogen occurs naturally as a diatomic, N2, the molar mass of
nitrogen gas is two times 14.01
g or 28.02 g/mol.
Calculating
Molar Mass
The
molar mass of a substance is the sum of the molar masses of each element.
What
is the molar mass of copper(II) nitrite, Cu(NO2)2?
The
sum of the atomic masses is as follows:
63.55 + 2(14.01 + 16.00 +
16.00) =
63.55 + 2(46.01) = 155.57 amu
per molecule
The
molar mass for Cu(NO2)2 is 155.57 g/mol.
Homework:
Using a periodic
chart calculate the molar mass of the following:
1. Calculate the molecular
mass of Acetic Acid, HC2H3O2.
2. Calculate the
formula unit mass of Ammonium Chromate,
(NH4)2CrO4 .
3. Calculate the
molecular mass of glucose,
C6H12O6.
Interactive Online Chem-i-Calc(Molar
Mass & % Composition):
http://people.emich.edu/bramsay1/ccc-release/chem.html
(Requires
JAVA)
Chapter
9 Part D Study Packet
Part D: Mole calculation
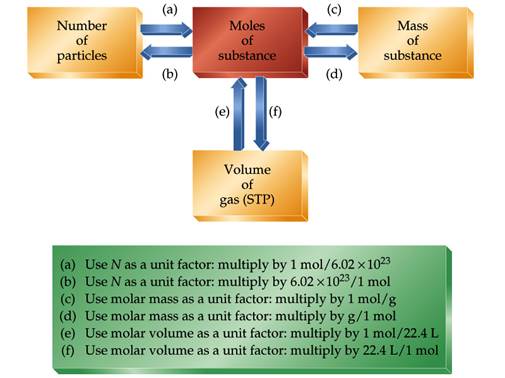
This is the only time in the course you will need to use Avogadro's number to relate actual particles to masses and volumes. In the lab we move from masses to mole and never focus on the number of particles.
What
is a mole?
The mole (mol) is a unit of measure
for an amount of a chemical substance (chemist dozen).
A mole is Avogadros number of particles,
which is 6.02 x 1023 particles.
1 mol = Avogadros number = 6.02 x 1023 units
(atoms, Molecules, ions, e1-)
We can use the mole relationship to convert between the number
of particles and the mass of a substance
Example
B1: How many sodium atoms are in 0.240 mol Na?
Question :
_____?______Na Atoms = 0.240 mol Na
1.
We want atoms of Na.
2.
We have 0.240 mol Na.
3.
1 mole Na = 6.02 x 1023 atoms
Na.
Solution:
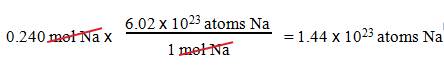
Homework #1:
B1: How many Carbon atoms are contained in 0.01667 moles
of Carbon ( 1 carat diamond)?
Example
B2: How many moles of aluminum are in 3.42 x 1021 atoms Al?
Question : _____?______Mole
Al = 3.42 x 1021 atoms Al
1.
We want moles Al.
2.
We have 3.42 x 1021 atoms Al.
3.
1 mol Al = 6.02 x 1023 atoms
Al.
Solution:
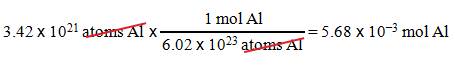
Homework #2:
B2: How many moles of Carbon are contained in 3.016 x 1022
atoms of Carbon (3 carat diamond)?
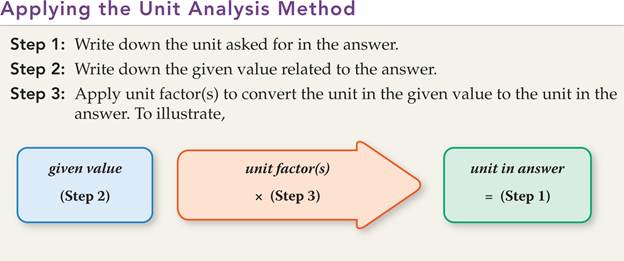
Part D1: Mole calculation II
We will be using the unit analysis method
again.
Recall the following steps:
Mole Calculations II
Now we will use the molar mass of a
compound to convert between grams of a substance and moles or particles of a
substance.
6.02 x 1023
particles = 1 mol = molar mass
If we want to convert particles to mass,
we must first convert particles to moles, and then we can convert moles to
mass.
Example B3: What is the mass of 1.33
moles of titanium, Ti?
1. We
want grams.
2. We
have 1.33 moles of titanium.
3. Use
the molar mass of Ti: 1 mol Ti = 47.88 g Ti.
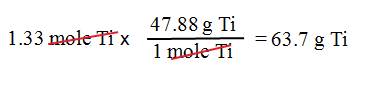
AtomsMass Calculation
Example B4: What is the mass of 2.55 x 1023
atoms of lead?
1. We
want grams.
2. We
have atoms of lead.
3. Use
Avogadros number and the molar mass of Pb.
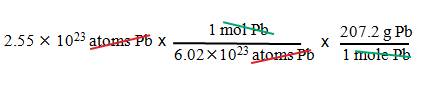
= 87.9 g Pb
MassMolecule Calculation
Example B5: How many F2
molecules are present in 2.25 g of fluorine gas?
1. We
want molecules F2.
2. We
have grams F2.
3. Use
Avogadros number and the molar mass of F2.
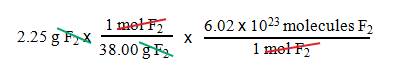
= 3.56 x 1022
molecules F2
Mass of an Atom or Molecule
What is the mass of a single
molecule of sulfur dioxide? The molar
mass of SO2 is 64.07 g/mol.
We want mass/molecule SO2,
we have the molar mass of sulfur dioxide.
Use Avogadros number and the molar
mass of SO2 as follows:
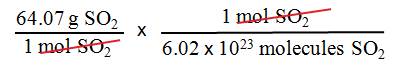
= 1.06 x 1022 g/molecule
Homework #3:
Calculate the mass in grams of 1.16 x 1022 molecules of nitrogen gas, N2
Homework #4:
Calculate the number of entities in 0.641 g of oxygen gas, O2
Part D2:
Percent Composition
The percent composition of a compound lists the
mass percent of each element.
For example, the
percent composition of water, H2O, is 11% hydrogen and 89% oxygen.
All water contains 11% hydrogen and 89% oxygen by mass.
Calculating Percent Composition of water
There are a few steps to calculating the percent
composition of a compound. Lets
practice
using H2O.
- Assume you have 1 mol
of the compound.
- One mole of H2O
contains 2 mol of hydrogen and 1 mol of oxygen. Therefore,
2(1.01
g H) + 1(16.00 g O) = molar mass H2O
2.02
g H + 16.00 g O = 18.02 g H2O
- Next, find the percent
composition of water by comparing the masses of hydrogen and oxygen in
water to the molar mass of water.
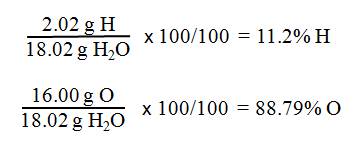
- Remember: g H/100 g H2O is % H and
g O/100 g H2O is % O
Example #2: Percent Composition
Problem
TNT (trinitrotoluene) is a white
crystalline substance that explodes at 240 °C. Calculate the
percent composition of TNT, C7H5(NO2)3.
First
calculate the molar mass of TNT
7(12.01 g C)
+
5(1.01 g H)
+
3 (14.01 g N + 32.00 g O) = g C7H5(NO2)3
84.07 g C
+ 5.05 g H
+
42.03 g N
+
96.00 g O
227.15 g C7H5(NO2)3
Second Calculate the % Composition
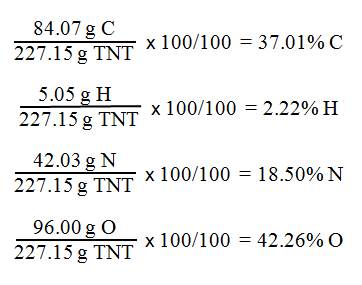
Homework:
Calculate the percent composition of each
element in the following compound:
Acetic
Acid HC2H3O2
There is an interactive web site that will allow you to check your
work for Parts A & C. The author of ChemiCalc (which I posses two site
licenses if you want a copy), Dr. Bert Ramsay of
http://people.emich.edu/bramsay1/ccc-release/chem.html
(requires JAVA)
Part D3:
Empirical Formula Calculation
What is the Empirical Formula?
The empirical formula of a
compound is the simplest whole number ratio of ions in a formula unit or atoms
of each element in a molecule.
The molecular formula of benzene is C6H6.
- The
empirical formula of benzene is CH.
The molecular formula of octane is C8H18.
- The
empirical formula of octane is C4H9
Calculating Empirical Formulas
from Lab Data
We can calculate the empirical formula of
a compound from its composition data upon analysis in the lab.
We can determine the mole ratio of each
element from the mass to determine the empirical formula of radium oxide, Ra?O?.
Example #1
A
1.640 g sample of radium metal was heated to produce 1.755 g of radium
oxide. What is the empirical formula?
We have 1.640 g Ra and 1.755 1.640
= 0.115 g O
The molar mass of radium is 226.03
g/mol, and the molar mass of oxygen is 16.00 g/mol.
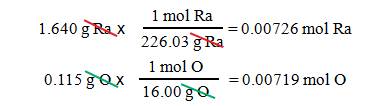
- We
get Ra0.00726O0.00719.
- Simplify
the mole ratio by dividing by the smallest number,0.00719 into both 0.00726 and
.00719.
- We
get Ra1.01O1.00
- Although
we use at three significant figures throughout the calculation, empirical
formulas are rounded to one significant figure resulting in whole number ratios
- Answer: RaO is the empirical formula
We will do a similar
calculation in the lab: Determining the Empirical Formula
Calculating Empirical Formulas
from % Composition
We can also use percent composition data
to calculate empirical formulas.
Assume that you have 100 grams of
sample.
Acetylene is 92.2% carbon and 7.83%
hydrogen. What is the empirical formula?
First:
If we assume 100 grams of sample,
we have 92.2 g carbon
and 7.83 g hydrogen
Second: Calculate the moles of each
element.
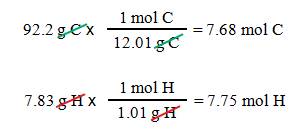
Third: The ratio of elements in acetylene
is C7.68H7.75.
Fourth:
Divide by the smallest number to get the following formula:
![]()
Homework #1 Empirical Formula via Lab Data:
In an experiment 2.410 gram of copper
oxide produced 1.925 g of copper metal after heating with oxygen gas. What is
the empirical formula of copper oxide?
Homework #2 Empirical Formula
via % Composition:
Given the following
percent compositions, determine the empirical formula of the following
compound: 54.5% carbon, 9.15% hydrogen, and 36.3% oxygen