CHM
1020 Path 4 Chapter 9 Study Pack – Part IV
G. ___ First Law of
Thermodynamics & Related Terms-
Answer
G1. ____ Discussion Questions-Chapter
9 Answer
G2. ____Specific Heat Problem-
Answer Sample 2
G3. ____Enthalpy Change with
Phase Change Prob- Answer Sample 2
H. ____ Enthalpy Change in Chemical
Reaction- Answer
H1. ___Bond Making/Breaking
Problem- Answer
H2. ____Introduction
to Entropy and Spontaneity-
Part G: First Law of Thermodynamics and Related Terms
Energy can be classified as kinetic and
potential. Kinetic energy is energy associated with motion, while Potential
energy is stored energy and can be converted to kinetic energy. The sum
of all the kinetic and potential energy in the universe is the Total Energy of
the Universe.
Examples of kinetic energy are:
Thermal Energy,
Mechanical Energy,
Radiant Energy,
Electrical Energy, and
Sound.
Examples of Potential Energy are:
Gravitational Energy,
Nuclear Energy,
Chemical Potential Energy, and
Electrostatic Energy.
You can go to the following web sites for
forms and conservation of energy:
http://www.eia.doe.gov/kids/energyfacts/science/formsofenergy.html
You should be able to identify forms of energy associated with energy changes.
What is the difference between heat and
temperature?
You
must understand the concept of an thermodynamic system
and its surroundings: :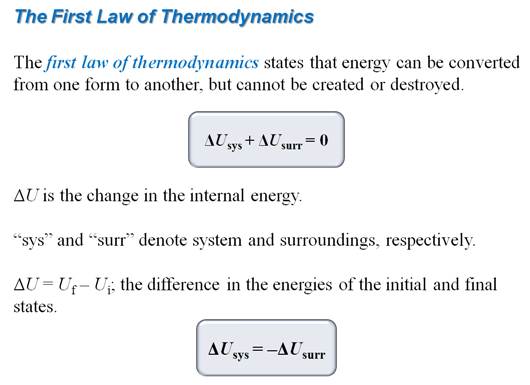
For Part A, you need to be able to
define the following:
1. State the first law of
thermodynamics:
The
energy of the universe is a constant. [Law of Conservation of Energy-Energy can
neither be created or destroyed, but can be converted from form to form]
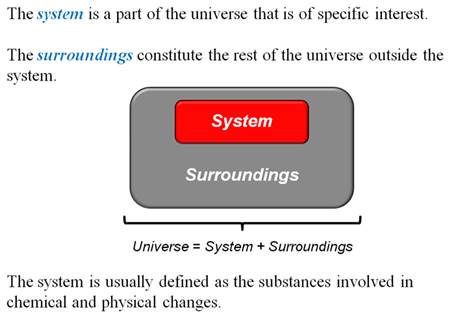
2. Explain a Thermodynamic System
and Its Surroundings
A thermodynamic
system is defined as the object, or collection of objects,
being studied.
The surroundings
include everything outside the system that exchange energy with
the system.
There
are three types
of systems: Open Systems; Closed Systems; and Isolated Systems:
Open Systems can gain or lose
energy across their boundaries.
Closed Systems can absorb or release
energy, but not mass, across the boundary. The mass of a closed system is a
constant, no matter what happens inside.
Isolated Systems cannot exchange matter
or energy with its surroundings. (Adibatic)
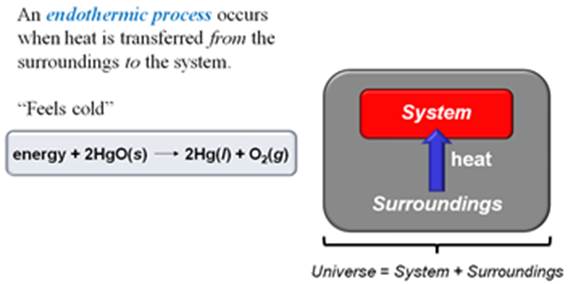
3. Define endothermic and exothermic
processes.
In the exothermic process heat is transferred from a
system to the surroundings.

An endothermic
process is the opposite of an exothermic process: heat is
transferred from surroundings of the system.
Chapter
9:
Part G First Law of Thermodynamics & Related
Terms
State the first law of thermodynamics:
Explain a Thermodynamic System and Its Surroundings:
Define endothermic and exothermic
processes:
State the second law of thermodynamics:
State the third law of thermodynamics:
Part G1: Discussion Questions
After studying the remainder of the
chapter you should be able to answer any two of the following discussion
questions:
1. What is the standard state of an element or compound substance and
give an example?
The standard
state of an element or a compound is defined as the most stable
form of the substance in the physical state that exists at a pressure of 1 bar
and specified temperature (usually 25oC or 298 K).
For example
∆Hof for CO2
(g):
At 25 oC and 1 bar, the standard state of
carbon is solid graphite, the most stable form of this element and the most
stable form of oxygen is O2 (g)
C(s) +
O2(g) à
CO2(g) ∆Hof = -393.5 kJ
2. Why does water have a high specific heat capacity? What does this mean?
The specific heat of water is much
larger than for most substances because of the unusually strong bonds between
the water molecules (Look up the hydrogen bond in later chapters). These
intermolecular bonds are progressively broken as more and more heat is added.
What this means is that a considerable quantity of heat is required to heat
water and considerable amount of heat must be transferred out of the water
before it cools down appreciably.
3. Write four different
mathematical expressions for the 1st Law of
Thermodynamics. How are they related?
Some expressions for the 1st
law are:
∆E = q + w
where ∆E
refers to the system
qin = qout
heat
gained = heat lost
∆E = zero
where
∆E refers in this case to the entire universe
∆Hºreaction = Σ(∆Hºf (products) - ∆Hºf (reactants) )
All these expressions represent an energy balance,
reflecting the fact that energy can neither be created nor destroyed.
4. Where does the energy come from in
an endothermic process? And where does it go?
In an endothermic process energy is
required. There are two sources for this energy: the energy may come from the
surroundings if the system is heated; or the energy could come from the system itself
if the kinetic energy of the atoms and molecules of the system is reduced. In
this case, the temperature of the system decreases. Unless the system is
isolated (well-insulated), there will be a movement of the energy between the
system and its surroundings to reestablish thermodynamic equilibrium.
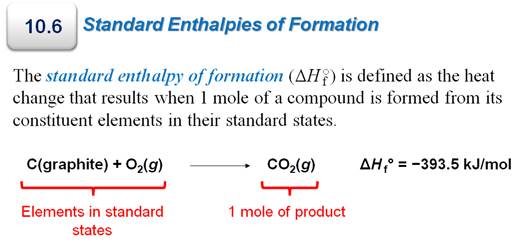
5. Define standard molar enthalpy of formation ∆Hºf.
Why is the standard enthalpy of formation
of a pure element in its most stable form defined as zero (page 284 and
see table 6.2 p 285)?
The standard molar enthalpy of formation of a
substance is the enthalpy change for a reaction in which one mole of the
substance in its standard state is made from its constituent elements in their
standard states.
For a substance that is an element,
such a reaction represents no change, and therefore then enthalpy change must
be zero because the element (or atom) already exists in nature and can not be assembled by man from its building blocks of
subatomic particles. Elements are defined as the smallest unit of matter that
has the chemical properties of that matter. It cannot be subdivided into it
building blocks by any chemical means. Therefore, we state energy change begins
with putting atoms together to make molecules of compounds.
6. Define a spontaneous
reaction. How can you tell whether a reaction is spontaneous?
A spontaneous reaction is a
reaction that happens by itself. It may happen quickly or very very slowly but it does happen. Calculations in
thermodynamics can be done to determine whether or not a reaction is
spontaneous. However, if a process or reaction does happen itself, you can be certain it is
spontaneous.
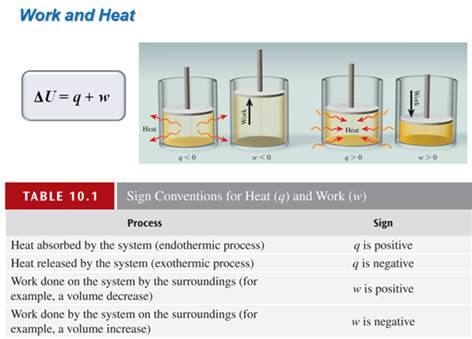
7. A system can exchange energy with its surroundings either by transferring heat or by doing work. This is expressed by the following equation: Δ E = q + w
Fill in the chart with correct signage:
Change Sign Conversion Effect of Esystem
Work done on the system by surroundings w > 0 (+) E increases (+)
Work done by the system on surroundings w < 0 (-) E decreases (-)
Heat transferred to system from surroundings q > 0 (+) E increases (+)
Heat transferred from system to surroundings q < 0 (-) E decreases (-)
Chapter 9:
Part G1 Discussion Questions
For the exam, your instructor will select four of the
following questions for you to write the answers:
1.
What is the standard
state of an element or compound substance?
2.
Why does water have a high specific heat capacity? What does this mean?
3.
Write four different mathematical
expressions for the 1st Law of Thermodynamics. How are they related?
4.
Where does the energy come from in
an endothermic
process? And where does it go?
5. Define standard
molar enthalpy of formation ∆ Hºf . Why is the standard enthalpy of formation of a pure element
in its most stable form defined as zero?
6.
Define a spontaneous reaction. How can you tell
whether a reaction is spontaneous?
7.
A system can exchange energy with its surroundings either by transferring heat
or by doing work. This is expressed by the following equation: Δ
E = q + w
Fill
in the chart with correct signage:
Change
Sign Conversion Effect of Esystem
Work done on the system by
surroundings
Work done by the system on
surroundings
Heat transferred to system from
surroundings
Heat transferred from system
to surroundings
Part C: Specific Heat Problem
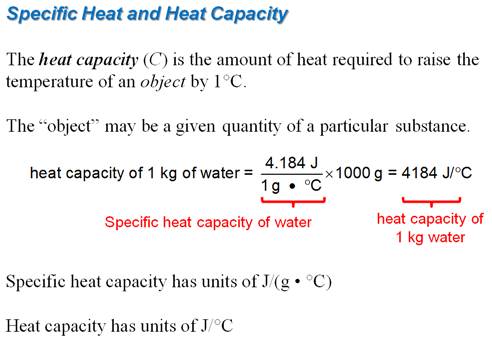
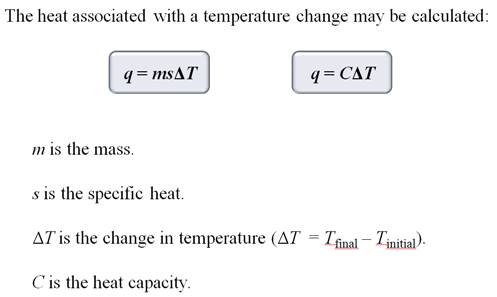
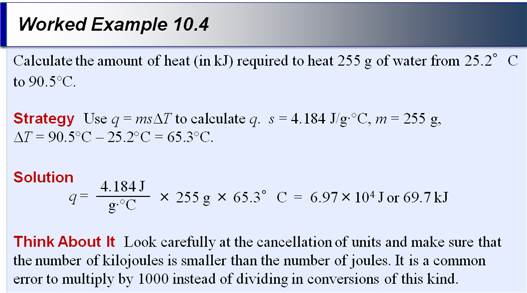

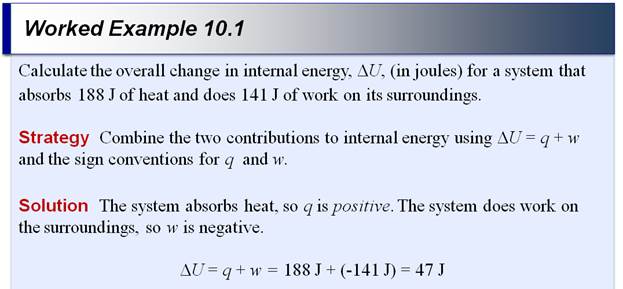
If the internal energy of a
thermodynamic system is decreased by 300 J when 75 J
of work is done on the system, how much heat was transferred, and in which
direction, to or from the system. See section 6.4 p 253
Change
Sign
Conversion Effect of Esystem
Work done on the system by
surroundings w > 0 (+)
E increases
Work done by the system on surroundings w < 0 (-) E decreases
Heat transferred to system from
surroundings q > 0 (+) E
increases
Heat transferred from system to
surroundings q < 0 (-) E decreases
Δ E = q + w
Given Δ E= - 300 J w = + 75 J
-
300J = q + (+75J)
q =
- 375 J of heat was transferred from the system to the surroundings
Chapter
9
Part
G2: Specific Heat/First Law Problems
If the temperature of a 50.0 gram block
of aluminum increases by 10.9 K when heated by 500 joules, calculate the:
- heat capacity of the aluminum
block.
- molar heat capacity of aluminum.
- specific heat capacity
of aluminum.
If the internal energy of a
thermodynamic system is decreased by 300 when 75 J of
work is done on the system, how much heat was transferred, and in which
direction, to or from the system
Answers:
a. heat capacity of the
aluminum block = 45.9 J/K
b. molar heat capacity of
aluminum 24.8 J/K mol
c. specific heat capacity of aluminum = 0.917
J/Kg
-375 J was transferred From the system
Part G3: Enthalpy Change with Phase Change
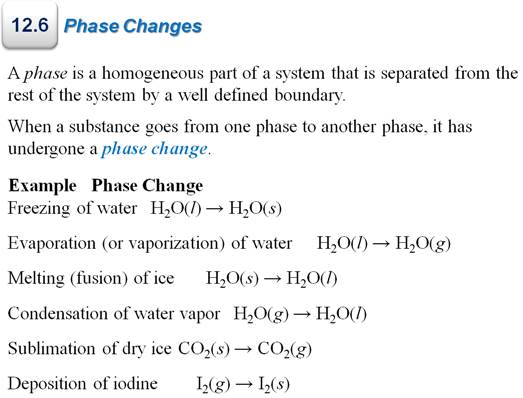
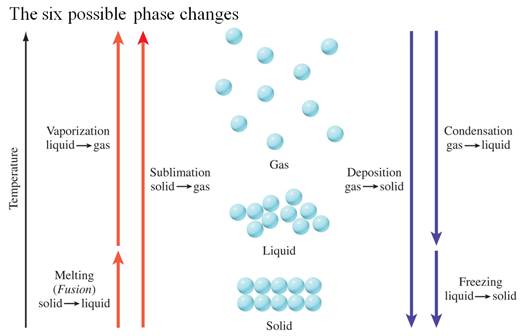
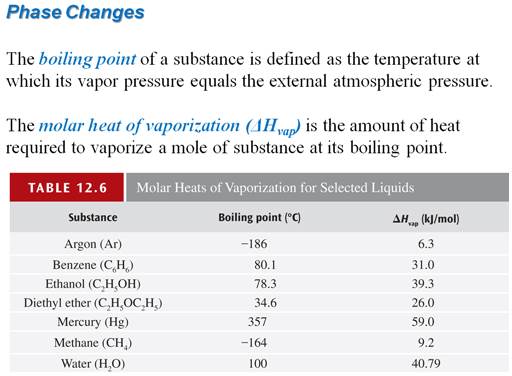

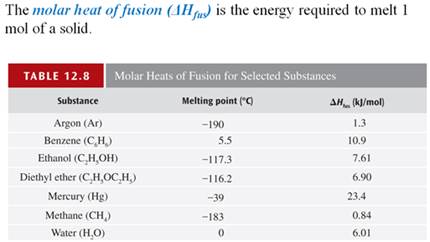
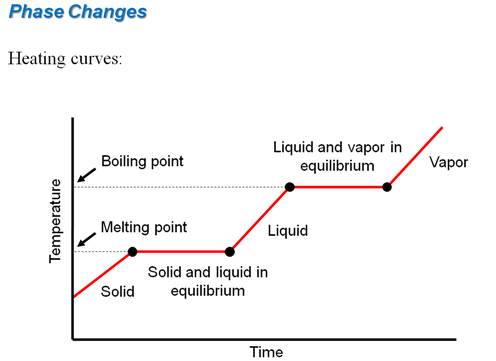
Chapter
9
Part
G4: Enthalpy change with Phase Change/Ice Cube Problem
Phase
Change:
1.
Calculate the amount of heat necessary to melt 27.0 grams of ice at 0oC, if the heat of fusion of ice is 333 J/g.
If I had the same amount of water at 100oC,
calculate the amount of heat required to boil 27.0 grams of water if the heat of vaporization of water is 2256 J/g?
How much heat is required to raise the
temperature of the 27 grams of water at
0oC to 100oC, if the specific heat of water is 4.184 J/goC
Ice
Cube Problem:
If 27.0 grams of ice at 0oC is added
to an insulated cup of water containing
123 grams of water at 50oC. What will be the final thermodynamic
equilibrium temperature of the water/ice mixture assuming no heat is lost to
the surroundings?
Part G5: Enthalpy Change in Chemical Reaction
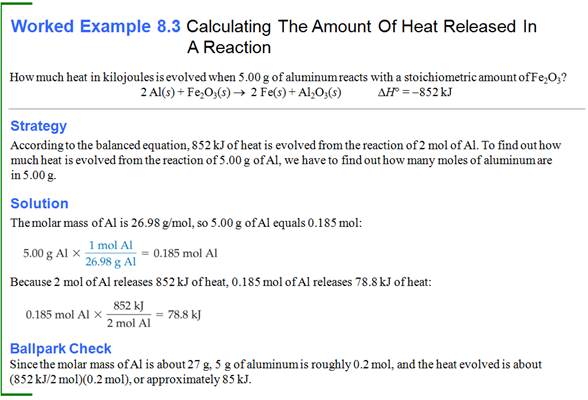
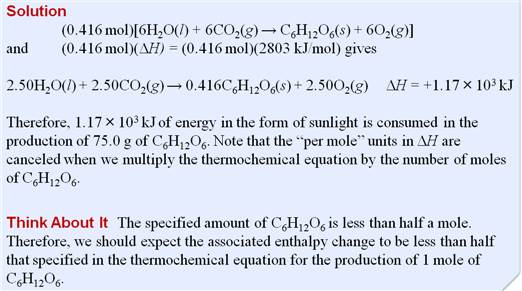
Chapter
9
Part
G4: Enthalpy change
in Chemical Reactions
If the enthalpy
change for the combustion of propane
gas, C3H8 (g) is -2220kJ/mol propane. What quantity of heat is released when
1.00 kg of propane is burned?
C3H8
(g) + 5 O2 (g) à 3 CO2 (g) + 4 H2O
(l) ∆H
= -2220 kJ
Answer:
-50,500 kJ
Part G0: Bomb/Coffee Cup Calorimeter
Problem:

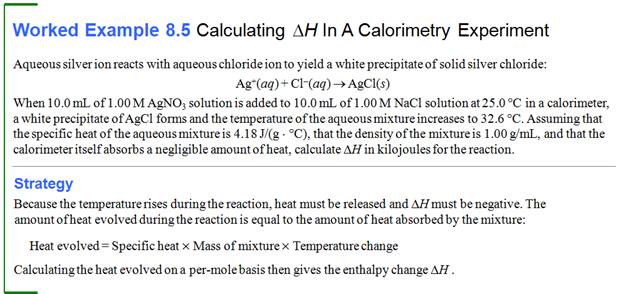
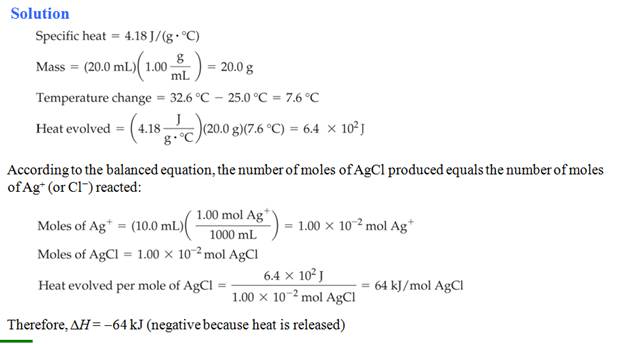
Chapter
9
Part G0: Calorimeter Problems
Benzoic acid (C6H5COOH) is sometimes used as a standard to
determine the heat capacity of a bomb calorimeter (constant volume). The
calorimeter is an insulated containing with 1.20 kg of water. When 1.32 g
of benzoic acid is burned in a calorimeter that is being calibrated
, the temperature rises from 20.93
oC to 22.93 oC.
What is the heat capacity of the calorimeter? The heat of combustion of
benzoic acid (qv) is -26.42 kJ/g
capacity of the calorimeter? The heat of
combustion of benzoic acid (qv) is -26.42 kj/g
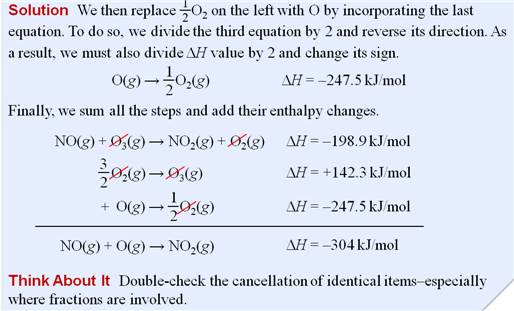
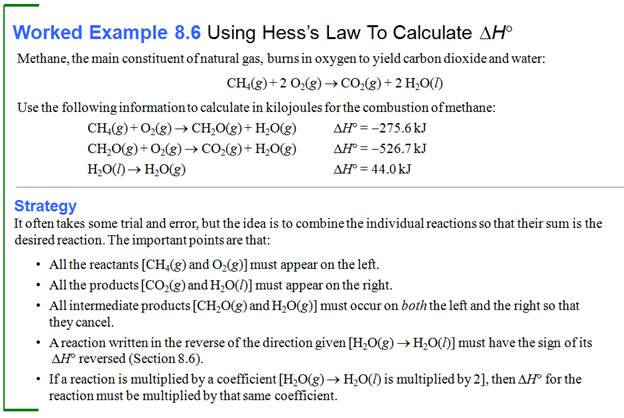
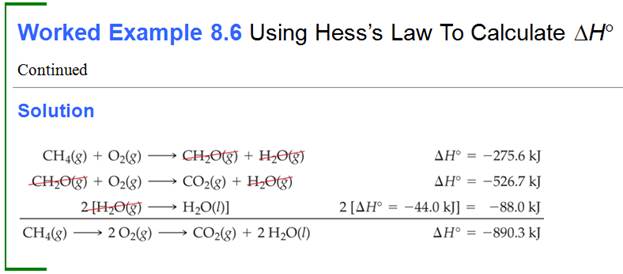
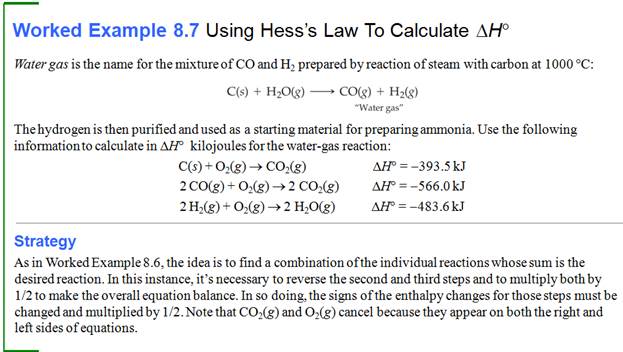
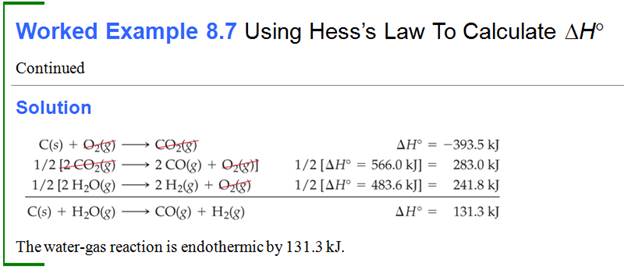
Part G5: Hess Law /Heats of Reaction Problems
My favorite problems for Chapter 9 involve Hess's Law. Hess's Law states : if a reaction is the sum of two or more other
reactions, then ΔH for the overall process is the sum of the ∆H values for those
reactions.
The interesting part of these
problems is to look at a set of reactions, two, three, four, or five. Then look
at the desired reaction for which the ∆H is unknown. The fun part is these sets of reactions
are kind of a puzzle maze.
You can do two things to a reaction to help you solve the problem.
1. You can rewrite the problem by writing the reverse reaction, making
the products now the reactants and the reactants now the products. All you have to so is change the sign of ∆H.
2. You can multiple any reaction through by a coefficient or a fraction,
and all you have to do is also multiple that ∆H by the same coefficient or fraction.
Once you have rearranged the equations in a set, they should add up to
the unknown or net equation.
Study example 6.8 on page 281 and work Exercise 6.19 on page 282-3.
Every college chemistry text has a neat set of Hess's Law problems. Our text
has ten examples at the end of the chapter Problems #6.79-6.88 on pages
296-297.
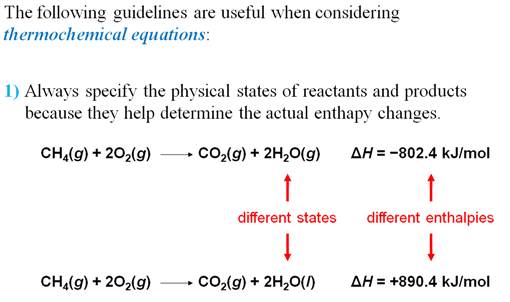
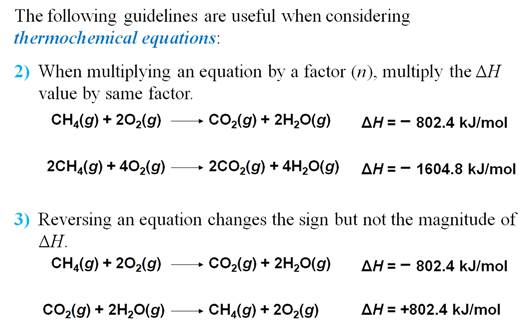
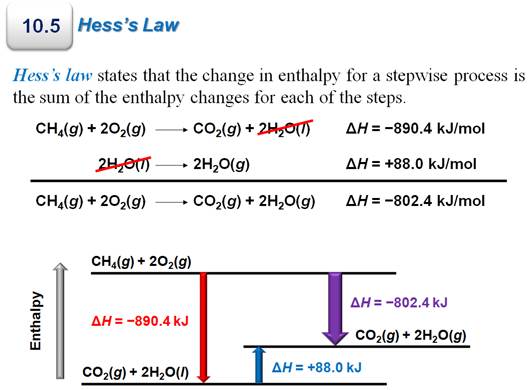
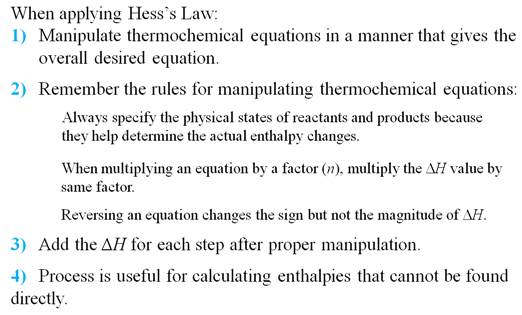


Chapter
9
Part
G5: Hess Law of Constant Heat Summation
Using
the following equations:
S (s) +
3/2 O2 (g) → SO3 (g) ∆
Ho = -395.2 kJ
2 SO2 (g) +
O2 (g) → 2
SO3 (g) ∆ Ho
= -198.2 kJ
calculate the ∆ Ho for the reaction:
S
(s) +
O2 (g) → SO2 (g)
Given
the following equations:
B2O3 (s) +
3H2O (g) → B2
H6 (g) + 3 O2
(g) ∆ Ho = +2035 kJ
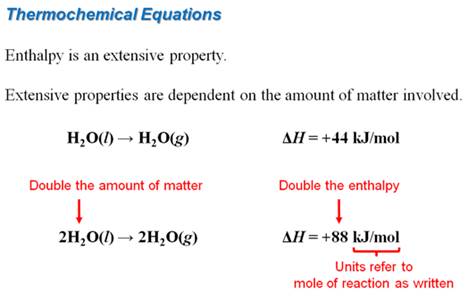
H2O (l) → H2O (g) ∆ Ho
= +44 kJ
2 B (s)
+ 3 H2 (g) →
B2 H6
(g) ∆ Ho
= +36 kJ
H2 (g)
+ 1/2 O2
(g) → H2O (l) ∆
Ho = -286 kJ
Calculate
the ∆ Ho for the reaction:
2 B (s) +
3/2 O2 (g)
→ B2 O3 (s)
Answer: -1273 kJ
Chapter 9
Part
G5: Hess Law of Constant Heat Summation
Problem#3
Using the following
equations (if necessary)
2CH4 (g) +
3 O2 (g) à 2
CO (g) + 4 H2O (l) ∆H˚ = -1215 kJ
2C (s) +
O2 (g) à 2 CO (g) ∆H˚ = -221 kJ
C (s) +
O2 (g) à CO2 (g) ∆H˚ = -394 kJ
to calculate the
enthalpy change for the reaction
CH4 (g) +
2 O2 (g) à CO2 (g) + 2 H2O (l) ∆H˚ = ?
Part G6: Standard Enthalpies of Formation Problem-
We need to introduce the concept standard
molar enthalpies of formation and the standard state. Always an interesting
question for the discussion board is a statement for the discussion board is:
The standard enthalpy of
formation for an element in its standard state is defined as ZERO. Why?
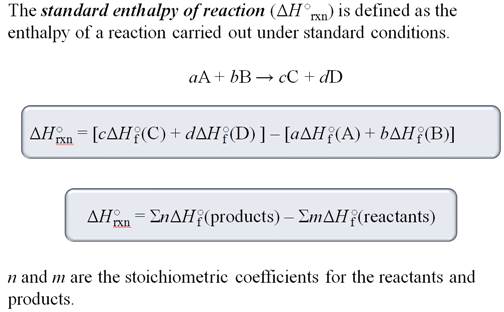
To calculate the enthalpy change for a
reaction you will use the following formula:
∆H˚rxn = ∑[∆H˚f (products)]- ∑[∆H˚f (reactants)]
Look closely at the problems. The trick is
always information seems to be left out. The standard enthalpies for formation
of elements are never given in the problem because its value is ZERO. Don't be
fooled. Also be careful about you signs as you make
you summations.
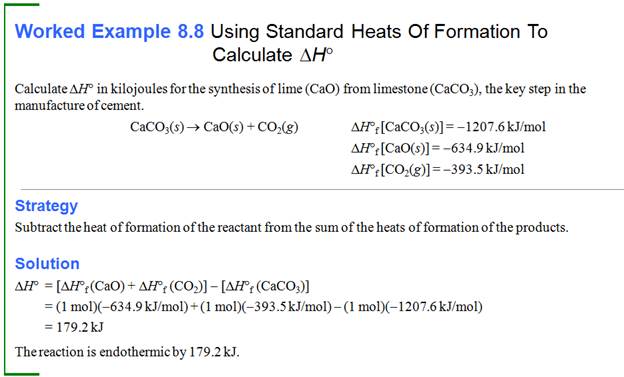
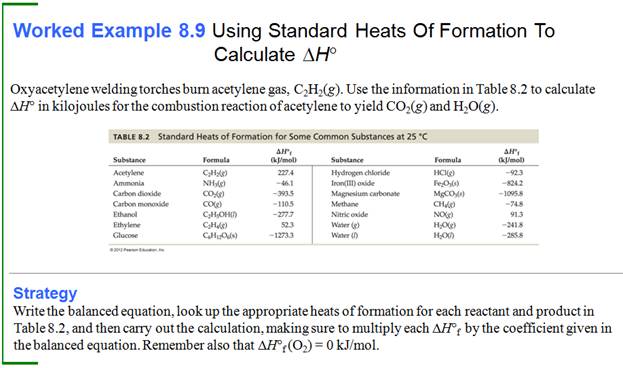
Part H: Standard Enthalpies
of Formation
Calculate ∆ H for the reaction:
2 Al (s) + 1 Cr2O3
(s) à 1 Al2O3 (s) + 2 Cr (s)
∆H˚f
(Al2O3 (s) ) = -1676 kJ/mol ∆H˚f (Cr2O3 (s) ) = -
1128 kJ/mol
Answer: -548 kJ
Chapter 9:
Part G6:
Standard Enthalpies of Formation continued
When ammonia is
oxidized to nitrogen dioxide and water, the quantity of heat released equals
349 kJ per mole of ammonia:
2NH3 (g) +
7/2 O2 (g) à 2
NO2 (g) + 3 H2O
(l) ∆H˚ = -698 kJ
Calculate the standard molar enthalpy of
formation of ammonia if
∆H˚f
(H2O(l) ) = -286 kJ/mol ∆H˚f
(NO2(g) ) = + 33 kJ/mol
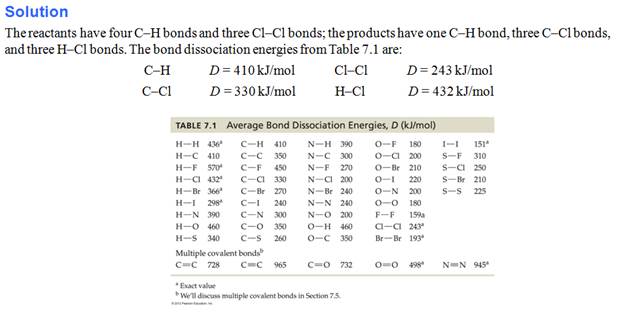
Part I: Bond Making/Breaking Problem-
In addition to the several calculations
above where you are trying to find the Enthalpy of Reaction, there is another
method which is explained in a video in Chapter 9.
Since the author assumes you do not know Dot
Structures of Molecules (which you do from Chapter 6). There is table which
gives the 'average' bond energies for many types of covalent bonds, especially
organic molecules. The skill need here is to be able to sketch the dot
structure (stick structure is ok) of each reactant and each product. The
formula which you will use to calculate the heat of reaction:
ΔHorxn= Σ (bonds broken) –
Σ (bonds formed)
.
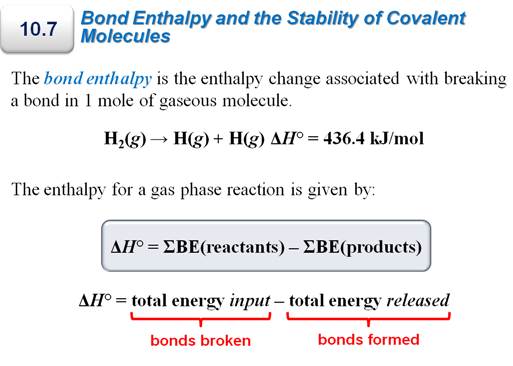

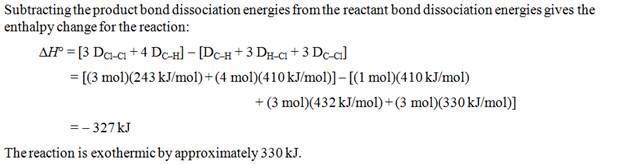
Chapter
9 Part G7: Heat of Reaction from Bond
Energies
Methane burns in oxygen to produce heat for homes by the following reaction:
CH4 +
2 O2 è CO2 + 2 H2O
If the average bond energies in kJ/mol are:
O-O 146
O=O 498
O-H 463
C-C 346
C-H 413
C-O 358
C=O 745
H-H 436
Calculate ∆Horxn for the reaction:
ΔHorxn = Σ (bonds broken) – Σ (bonds formed)
Chapter 9 continued
Part G7: Heat of
Reaction from Bond Energies
Propylene burns in
oxygen to produce heat by the following reaction:
2 C3H6 +
9 O2 è 6
CO2 + 6 H2O
If the average bond
energies in kJ/mol are:
O-O 146
O=O 498
O-H 463
C-C 346
C-H 413
C-O 358
C=O 745
H-H 436
C=C 134
ΔHorxn = Σ (bonds broken) – Σ (bonds
formed)
Calculate ∆Horxn for the reaction (Hint draw the
dot/stick structures of the compounds):
Chapter
9:
Part H:
Introduction to Entropy and Spontaneity-
What does entropy
measure?
How is it possible
for a reaction to be spontaneous yet endothermic?
Tell whether the entropy changes for the following process are likely
to be positive or negative?
(a) The fizzing of a newly opened can of soda?
(b) The growth of a plant from seed?
One of the steps in the cracking of petroleum into gasoline involves the
thermal breakdown of large hydrocarbon molecules into smaller ones. For example
the following reaction might occur:
C11H24 à C4H10 + C4H8 +
C3H6
Is
ΔS for this
reaction likely to be positive or negative?
Explain!
Chapter
9
Part H1:
Introduction to Free Energy and Spontaneity-
What are the two terms that makeup the free-energy change for the reaction, ΔG,
and which of the two is usually more important?
Tell whether reactions with the following values of ΔH and ΔS are spontaneous or non spontaneous and whether they are exothermic or
endothermic?
(a) Δ H = -48 kJ; ΔS = +135 J/K at 400K
(b) Δ H = -48 kJ; ΔS = -135 J/K at 400K
(c) Δ H = +48 kJ; ΔS = +135 J/K at 400K
(d) Δ H = +48 kJ; ΔS = -135 J/K at 400K