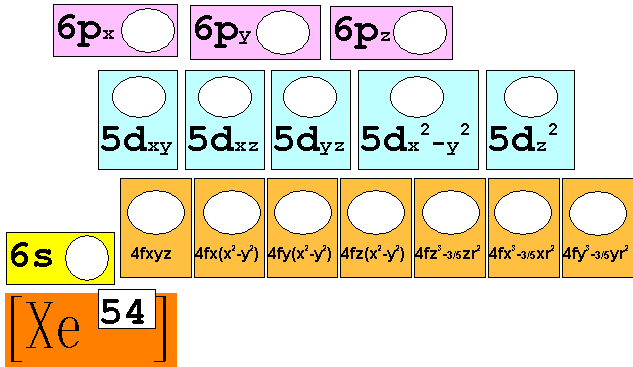Pathway 4: Chapter 4 Homework Pack
Chapter 4:
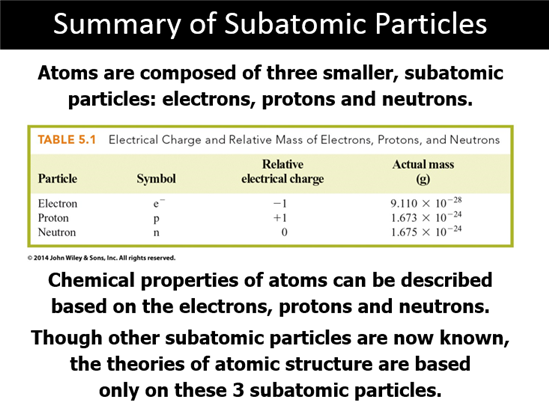
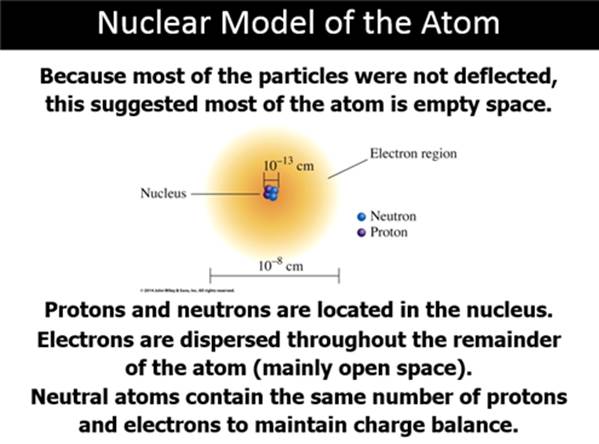
Subatomic Particles
Part S: Shapes of Orbitals
Part V: Chapter 4 Vocabulary Chapter 4 pages
123-124 †Answers
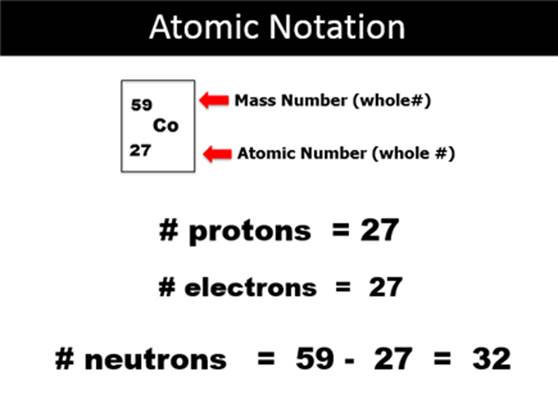
Module Three: Part A†† Atomic Notation
† 
††††††  †††††††††††††††††††††
†††††††††††††††††††††
Chapter 4 Part A: Atomic
Notation
Given the following
elements, atomic numbers, and mass numbers, State the number of electrons,
protons, and neutrons in the following elements:
1.†††† 23Na11†††††††††††††††††††††††† ††††††††††††Protons††† = ______
Electrons
= ______
Neutrons† = ______
2.†††† 93Nb41†††††††††††††††††††††††††††††††††††††
Protons††† = ______
Electrons = ______
Neutrons† = ______
3.†††† 20Ne10†††††††††††††††††††††††††††††††††††††
Protons††† = ______
Electrons = ______
Neutrons† = ______
4.†††† 59Ni28†††††††††††††††††††††††††††††††††††††††
Protons††† = ______
Electrons = ______
Neutrons† = ______
5.†††† 19F9†††††††††††††††††††††††††††††††††††††††††
Protons††† = ______
Electrons = ______
Neutrons† = ______
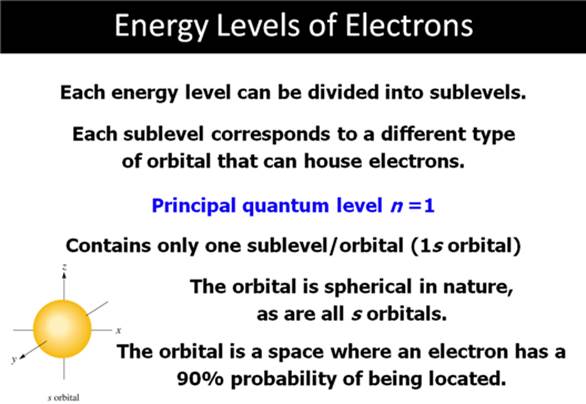
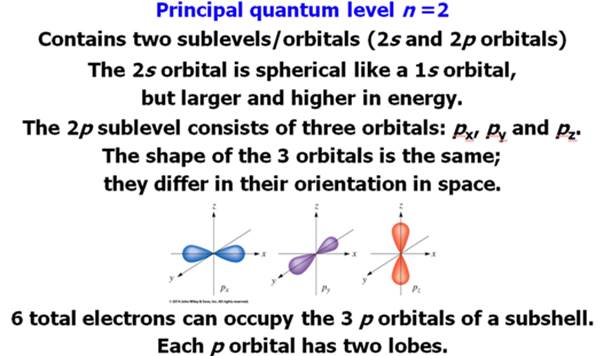
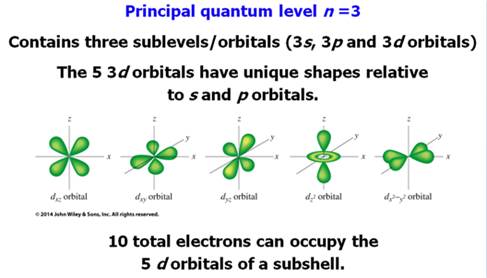
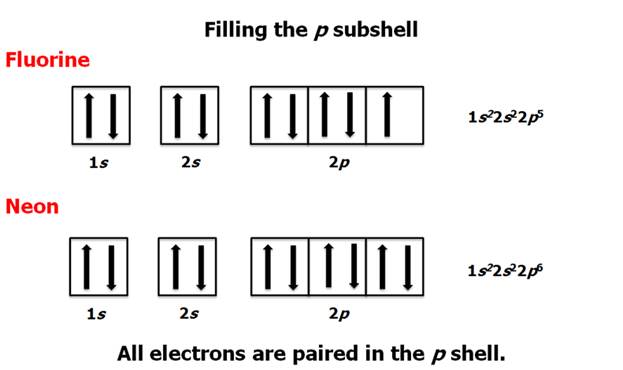
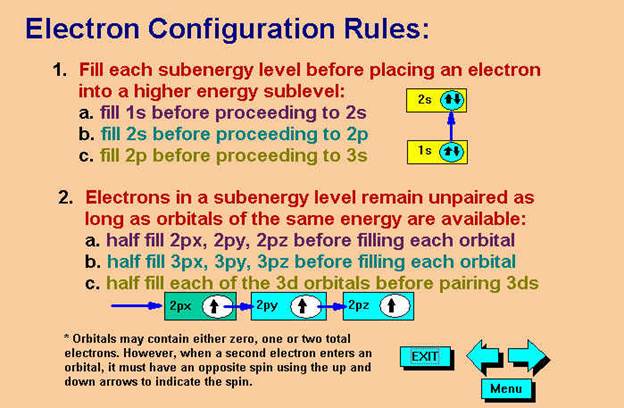
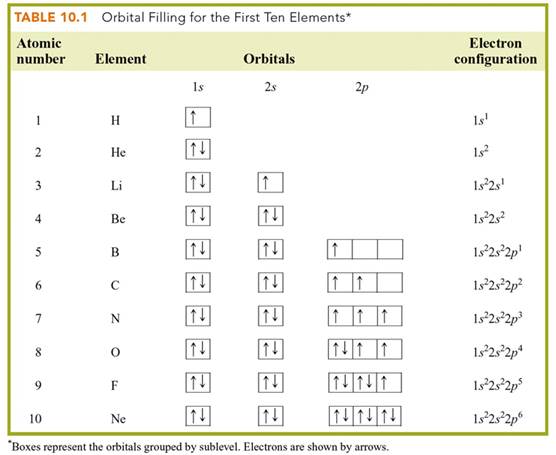
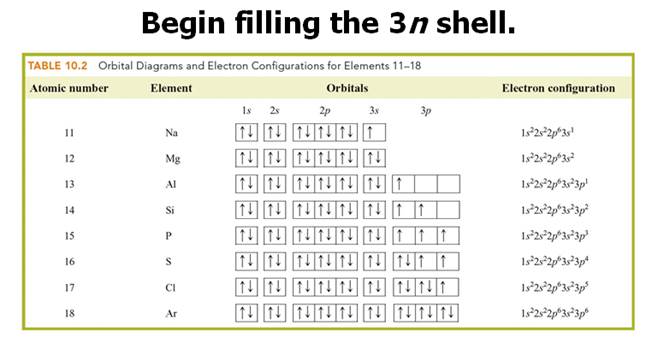
Chapter 4 Part B: Electron
Configuration

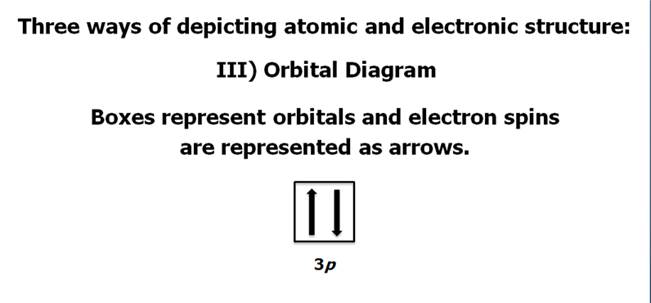
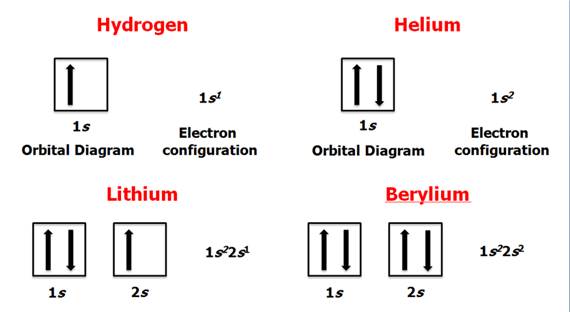
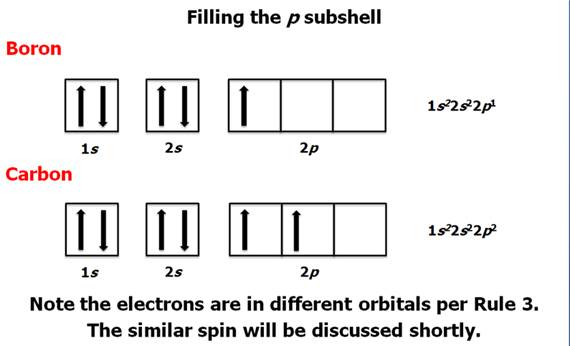

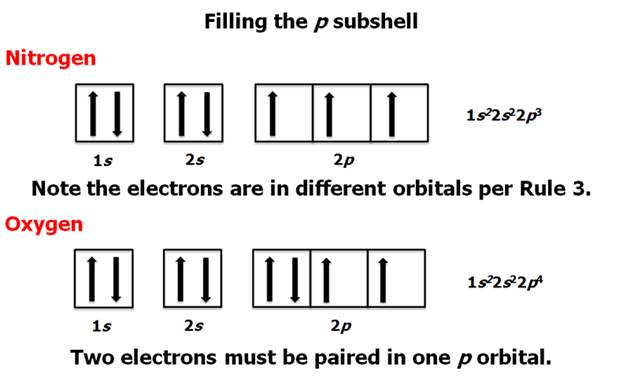
Interactive Electron Configuration Web
Site:
http://www.lsua.info/e_config/e-1instruct.html
Electron Configuration Rules Menu:
http://www.fccj.info/e_configMenu/e-1Menu.html
Check Your Answers. Click
on the element on the periodic table:
http://fscj.me/e-1Spectroscopic/pc.html
Animation of Elements
1-112 filling electrons:
http://www.northcampus.net/ElectronConfiguration/SpectroscopicNotation/spectroscopicNotation.html
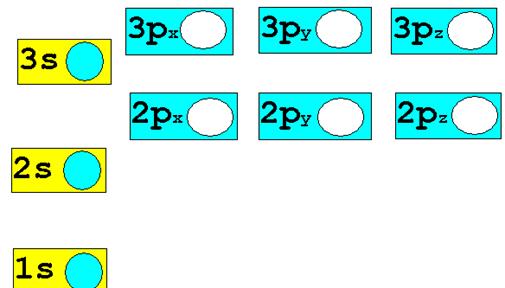
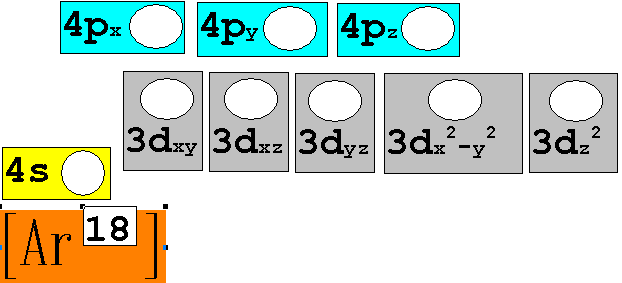
Chapter 4: Part B:††
Electron Configuration†††
Given the
following elements and atomic numbers, use arrows to fill-in the electron
configuration, then rewrite the configuration into the chemistís shorthand
(spectroscopic Notation):
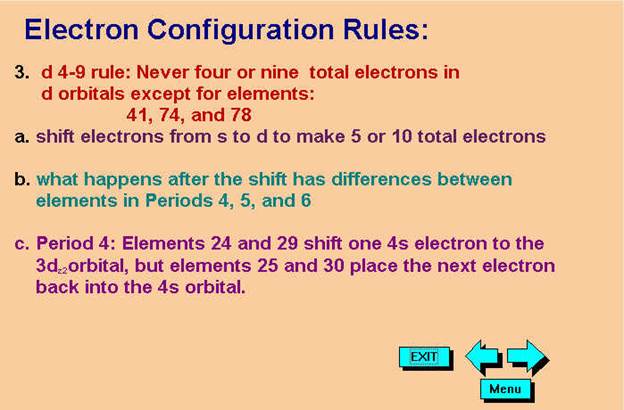
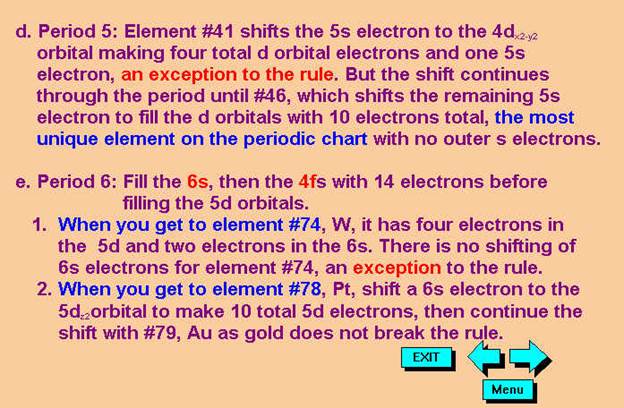
*remember 4/9 rule
exceptions: Never 4/9 total d orbital electrons except for elements 41, 74, 78.
1.† 12C6††††††††
Chemist Shorthand:_____________________________________
2. 60Co27†† Chemist Shorthand:_____________________________________
Chapter 4: Part B: Electron Configuration
continued:
3.† 40Ca20†††††††† Chemist Shorthand:____________________________

4.† 52Cr24†††††††† Chemist Shorthand:____________________________

Chapter 4: Part B: Electron Configuration
continued:
5.† 65Zn30†††††††† Chemist Shorthand:____________________________

6.† 85Nb41†††††††† Chemist Shorthand:____________________________
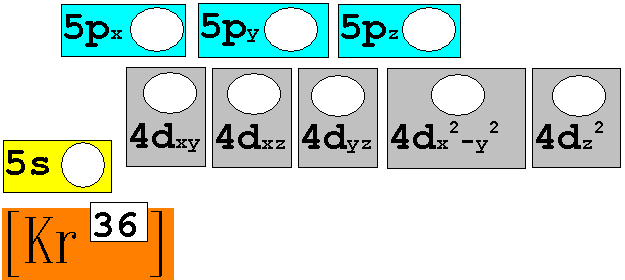
Chapter 4: Part B: Electron Configuration
continued:
7.† 96Mo42†††††††† Chemist Shorthand:____________________________

8.† 180Au79†††††††† Chemist Shorthand:____________________________
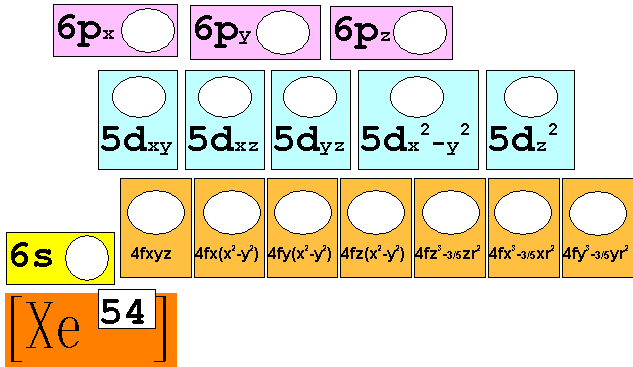
B#1.† Pd46†††††††† Chemist Shorthand:____________________________

B#2.† Pt78†††††††† Chemist Shorthand:____________________________
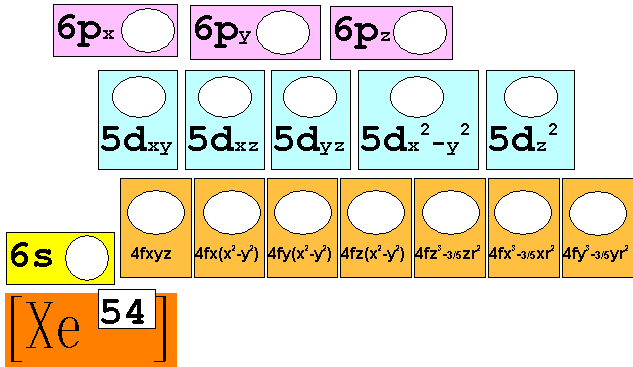
B#3.† Ag47††† †††††Chemist Shorthand:____________________________

B#4.† W74†††††††† Chemist Shorthand:____________________________
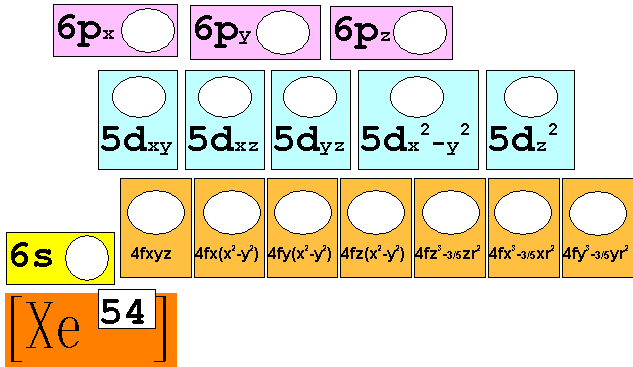
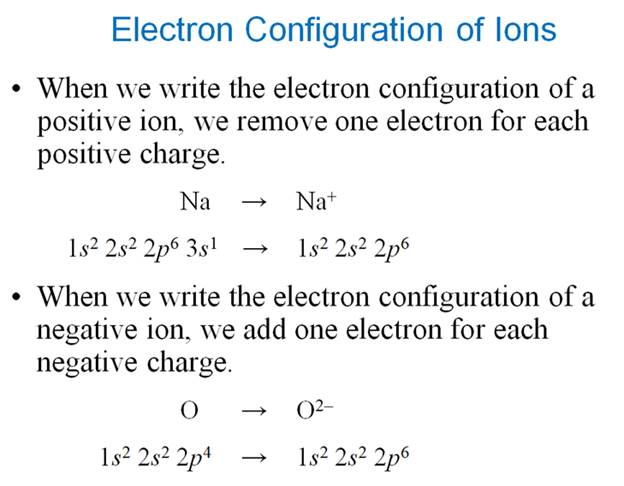
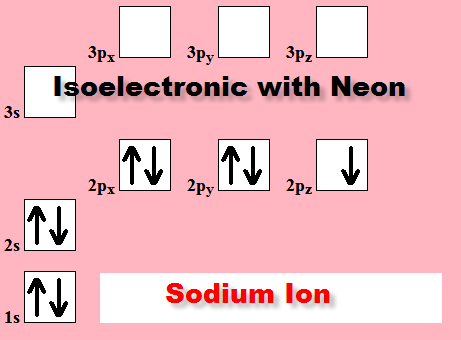
Chapter 4 Part B1:
Electron Configuration of Ions
†††††††††††† 
††††††††††† ††
Chapter 4 Part B1:††
Electron Configuration of Ions†††
Given the following
ions, use arrows to fill-in the electron configuration of the ion, then rewrite
the configuration into the chemistís shorthand:
1.†††† Cl1-† ion†††††
Chemist Shorthand: ___________________________
2.† K1+ ion† Chemist Shorthand:
_____________________________

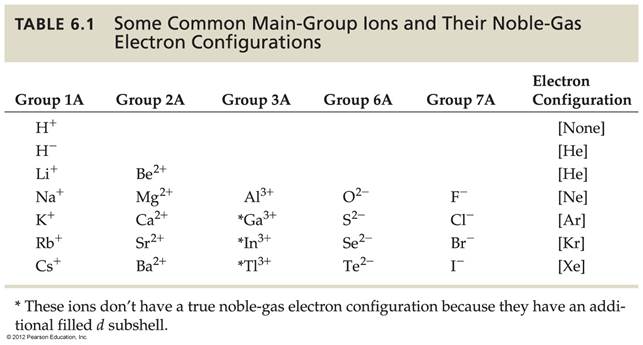
Remember positive ions have lost electrons from the
neutral atom, while negative ions have gained electrons into the neutral atom.
Negative Ions are always isoelectronic
with the Nobel Gas at the end of the period:
Nitride N3- Ion is isoelectronic with
the Neon atom
Oxide ††O2- Ion is
isoelectronic with the Neon Atom
fluoride F1- Ion is isoelectronic with the Neon Atom
Positive Ions are always isoelectronic with the Nobel
Gas which ends the
previous period:
Aluminum Al3+ Ion is isoelectronic with
the Neon atom
Magnesium Mg2+ Ion is isoelectronic with the Neon Atom
Sodium Na1+ Ion is isoelectronic with the Neon Atom
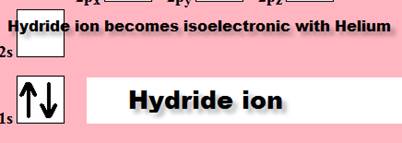
How about the Hydrogen atom which some classify as
an element in a class by itself CAN become an ion (two different ways).
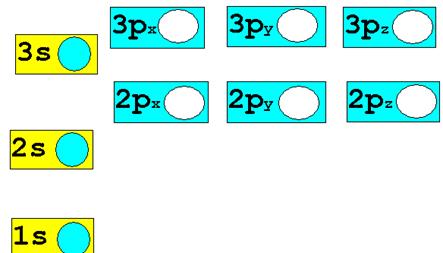
1.†††† H1-† ion†††††
Chemist Shorthand: ___________________________
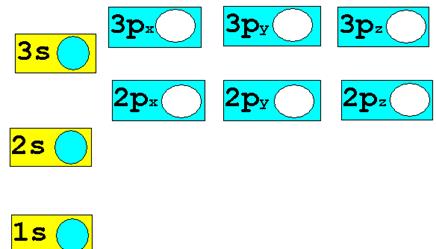
2.† H1+ ion ††Chemist
Shorthand: _____________________________
††††††††††††††††††††††††††††††††  ††
††
†††††††††††††††††††††††
†††††††††††††††††††††††††

Chapter 4:
Part C Orbital Subshells & Periodic Chart:
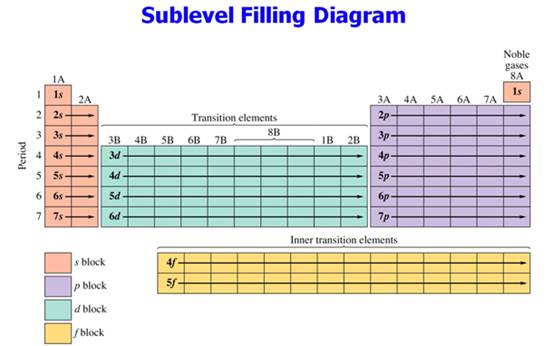
A
lot of students label the helium box 1p.
A
lot of students label the 3d block of elements as 4d!
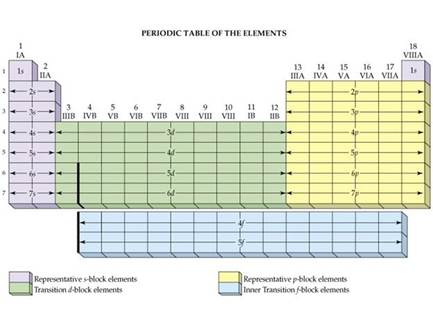
Chapter 4:
Part C Orbital Subshells & Periodic Chart Sample Exam
On the periodic chart below show all the s, p, d and f block elements on
the first six rows of the periodic table (Label each area beginning with 1s,
2s, 2p, etc):
|
periodic table
|
|
Group
|
1
|
2
|
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
11
|
12
|
13
|
14
|
15
|
16
|
17
|
18
|
|
Period
|
|
|
1
|
1
†
|
|
2
†
|
|
2
|
3
†
|
4
†
|
|
5
†
|
6
†
|
7
†
|
8
†
|
9
†
|
10
†
|
|
3
|
11
†
|
12
†
|
|
13
†
|
14
†
|
15
†
|
16
†
|
17
†
|
18
†
|
|
4
|
19
†
|
20
†
|
|
21
†
|
22
†
|
23
†
|
24
†
|
25
†
|
26
†
|
27
†
|
28
†
|
29
†
|
30
†
|
31
†
|
32
†
|
33
†
|
34
†
|
35
†
|
36
†
|
|
5
|
37
†
|
38
†
|
|
39
†
|
40
†
|
41
†
|
42
†
|
43
†
|
44
†
|
45
†
|
46
†
|
47
†
|
48
†
|
49
†
|
50
†
|
51
†
|
52
|
53
†
|
54
†
|
|
6
|
55
†
|
56
†
|
*
|
71
†
|
72
†
|
73
†
|
74
†
|
75
†
|
76
†
|
77
†
|
78
†
|
79
†
|
80
†
|
81
†
|
82
†
|
83
†
|
84
†
|
85
†
|
86
†
|
|
7
|
87
Fr
|
88
Ra
|
**
|
103
Lr
|
104
Rf
|
105
Db
|
106
Sg
|
107
Bh
|
108
Hs
|
109
Mt
|
110
Ds
|
111
Rg
|
112
Uub
|
113
Uut
|
114
Uuq
|
115
Uup
|
116
Uuh
|
117
Uus
|
118
Uuo
|
|
|
|
|
*Lanthanoids
|
*
|
57
†
|
58
†
|
59
†
|
60
†
|
61
†
|
62
†
|
63
†
|
64
†
|
65
†
|
66
†
|
67
†
|
68
†
|
69
†
|
70
†
|
|
|
|
**Actinoids
|
**
|
89
Ac
|
90
Th
|
91
Pa
|
92
U
|
93
Np
|
94
Pu
|
95
Am
|
96
Cm
|
97
Bk
|
98
Cf
|
99
Es
|
100
Fm
|
101
Md
|
102
No
|
|
|
Chapter 4 Part C1: Spectroscopic Notation from
Periodic Chart
Sample #1: Write the spectroscopic notation of the
Chlorine atom:
a. Chlorine is on the third row of the periodic table.
b. Neon is the Nobel Gas which ends the second period. Write [Ne] to begin the
answer.
c. Look at the Periodic Table and Count the squares Left to Right on the third
row to the chlorine atom.
d. write 3s2 after count 1,2
e. then 3,4,5,6,7 to Chlorine and write 3p5
Answer:† [Ne] 3s23p5
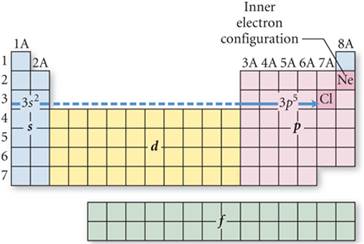
Sample #2: Write the spectroscopic
notation for Selenium atom:
On the fourth row of the periodic
table you have to include the 3d orbitals when counting from left to right: #1,2=4s#; †#3-12=3d10;
#13,14,15,16=4p4
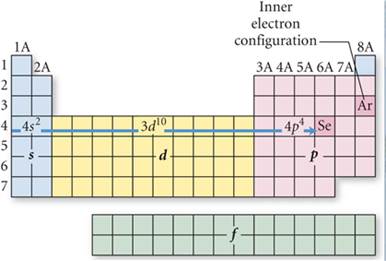
Answer:† [Ar] 4s23d104p4
Chapter 4 Part C1: Spectroscopic Notation from
Periodic Chart
|
1
|
IA
|
IIA
|
|
|
|
|
H
|
|
|
|
|
|
IIIA
|
IVA
|
VA
|
VIA
|
VIIA
|
He
|
|
2
|
|
|
|
|
|
† *
|
|
|
|
|
*
|
|
|
|
|
†
|
†
|
Ne
|
|
3
|
|
|
IIIB
|
IVB
|
VB
|
VIB
|
VIIB
|
VIIIB
|
|
|
IB
|
IIB
|
|
|
|
|
|
Ar
|
|
4
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Kr
|
|
5
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Xe
|
|
6
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rn
|
|
7
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
M-3
C1: Spectroscopic Notation using the Periodic Chart†
Given the
Elementís Atomic Number, use the Periodic Chart above to write the
Spectroscopic Notation for the following elements..
You may do it the long way showing all blocks of orbitals, or you may use the
shorter method applying the square brackets around the Nobel Gas which
indicates the complete inner filled electrons in the core (or Kernel).
i.e:† [Ar] represents† 1s2 2s2 2p6
3s2 3p6
or the 18 electrons in the Argon core.
* In
columns VIB and IB, you may have to apply the d4/9 Rule (Never 4/9 total d orbital electrons in any spectroscopic notation
except Nb 41; W 74; and Pt 78)
- 1H††††††††
__________________________________________
- 30Zn††††† __________________________________________
- 35Br††††††
__________________________________________
- 74W†††††††
___________________________________________
- 8O††††††††
___________________________________________
- 15P†††††††
___________________________________________
Chapter 4
Part
F1:† Electron Dot Structures using the
Periodic Table

††††††††††††††
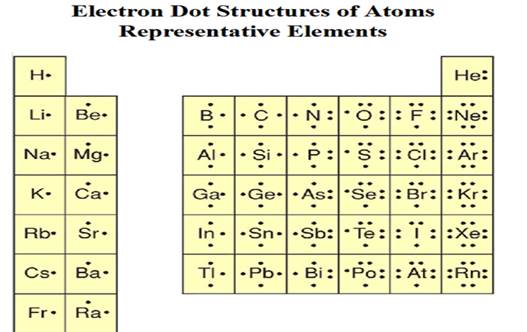 †
†
†††††††††††††††††††††††††††††††††††† Please
Note how Helium is different. Why?
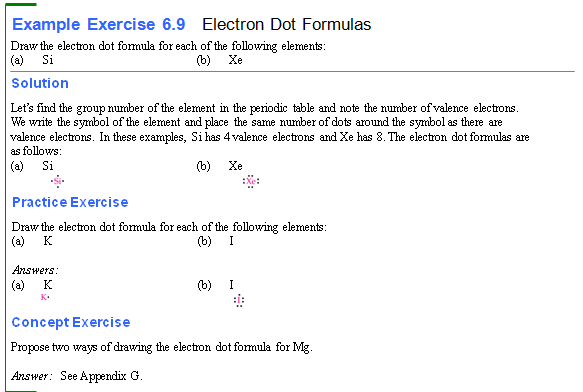
Chapter 4:
Part F1††††††† Electron Dot Formulas of
Atoms†††††††
Using the periodic chart, draw the electron
dot formulas of the following elements (the numbers shown are the elementís
atomic number and mass number):
1.† 6C12†††††††††††††††††††††††††††††††††††††††††††††††††††††††††† 6.†† 1H1
2.† 14Si28†††††††††††††††††††††††††††††††††††††††††††††††††††††††† 7.†† 7N14
3.†† 9F19†††††††††††††††††††††††††††††††††††††††††††††††††††††††††† 8.†† 8O16
4.† 11Na23†††††††††††††††††††††††††††††††††††††††††††††††††††††† 9.†† 10Ne20
5.† 15P31††††††††††††††††††††††††††††††††††††††††††††††††††††††††† 10.† 16S32
Chapter 4: Part F2 Periodic Ionic Character
†††††††††††† 
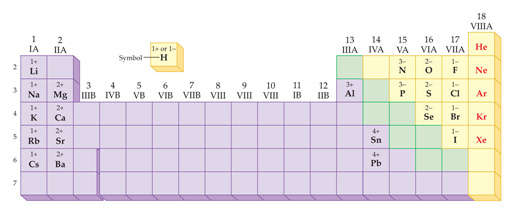
†††††††††††† 
Chapter 4:
Part F2†††††† Periodic Ionic
Properties††††††† †
Using a periodic chart, write the ionic character (monoatomic ionic
charge) of the following elements: (The number before the element is
its atomic number)
1.† 19 K†††† ________††††††††††† 6.††† 9F††††† _____
2.† 20Ca††† _______††††††††††††††††††††††††† 7.††† 1H††††† _____†††
_____††
3.† 7N††††††† _______†††††††††††††††††††††††† 8.††† 16S†††† _____
†
4.† 17Cl††††† _______†††††††††††††††††††††††† 9.††† 10Ne†† _____
5.† 53I† †††† †††______†††††††††††† ††††††† 10.††
15P†††† _____†††††††
Chapter 4 Part F: Periodic Properties
Same as Chapter 3 Part P
Pathway 4: Chapter
4 Vocabulary
Fill the blanks with word or the words that best fit the description
Reference Chapter 4 Page 123-124.†††††††††††††††† ††††††††
1._____________________†
The total mass of an atom. The atomic mass of each element presented in
the periodic table is the average
atomic mass of the various isotopes of that element occurring in nature.
2. _____________________The dense, positively charged
center of every atom.
3. _____________________The number of protons in the atomic
nucleus of each atom of a given element.
4. _____________________The pattern of frequencies of
electromagnetic radiation emitted by the atoms of an element, considered to be
an elementís ďfingerprint.Ē
5. _____________________A representation of a system that
helps us predict how the system behaves.
6. _____________________The nuclear charge experienced by
outer-shell electrons, diminished by the shielding effect of inner-shell
electrons and also by the distance from the nucleus.
7. _____________________The complete range of waves, from
radio waves to gamma rays.
8. _____________________†
An extremely small, negatively charged subatomic particle found outside
the atomic nucleus.
9. _____________________The arrangement of an atomís
electrons within orbitals.
10. _____________________A schematic drawing used to arrange
atomic orbitals in order of increasing energy levels.
11. _____________________The tendency of inner-shell
electrons to partially shield outer-shell electrons from the attractive pull
exerted by the positively charged nucleus.
12. _____________________The amount of energy needed to pull
an electron away from an atom.
13. _____________________ Any member of a set of atoms of
the same element whose nuclei contain the same number of protons but different
numbers of neutrons.
14. _____________________The number of nucleons (protons
plus neutrons) in the atomic nucleus. Used primarily to identify isotopes.
15. _____________________†
An electrically neutral subatomic particle found in atomic nuclei.
16. _____________________†
Any subatomic particle found in an atomic nucleus. Another name for
either proton or neutron.
17. _____________________A representation of an object on
some convenient scale.
18. _____________________ †A positively charged subatomic particle in
atomic nuclei.
19. _____________________ A small, discrete packet of
energy.
20. _____________________An integer that specifies the
quantized energy level within an atom.
21. _____________________ A graphic representation of a
collection of orbitals of comparable energy in a multielectron atom. A shell
can also be viewed as a region of space about the atomic nucleus within which
electrons may reside.
22. _____________________ A device that uses a prism or
diffraction grating to separate light into its color components and measure
their frequencies.


 †††††††††††††††††††††
†††††††††††††††††††††