Pathway
4: Chapter 7 Study Pack
Part
A: Properties of
Solutions
Answers
Part B: Dissolving Process
Part B1: Factors
Affecting Rate of Dissolving
Answers
Part C: Intermolecular Forces Answers
Part
D: Units
of Concentration of Solutions Answers
Part D1: Solution
Preparation Problems Answers
Part V: Chapter 7 Vocabulary
Part A: Properties of Solutions
Back in Chapter 3 a solution
was introduced as a homogeneous mixture of a solute and solvent and you
included them in your matter chart for Part G of Chapter 3. A solution is
defined as a homogeneous mixture of two or more substances. The two new words that are introduced are solute and solvent. Although we usually think of solutions to be
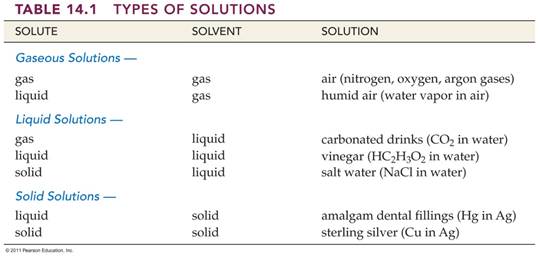
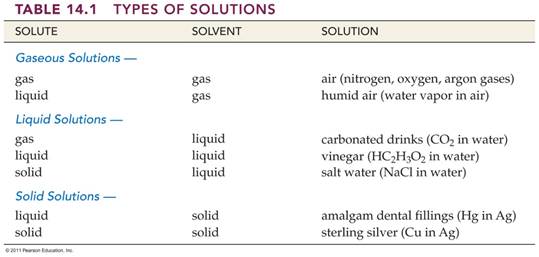
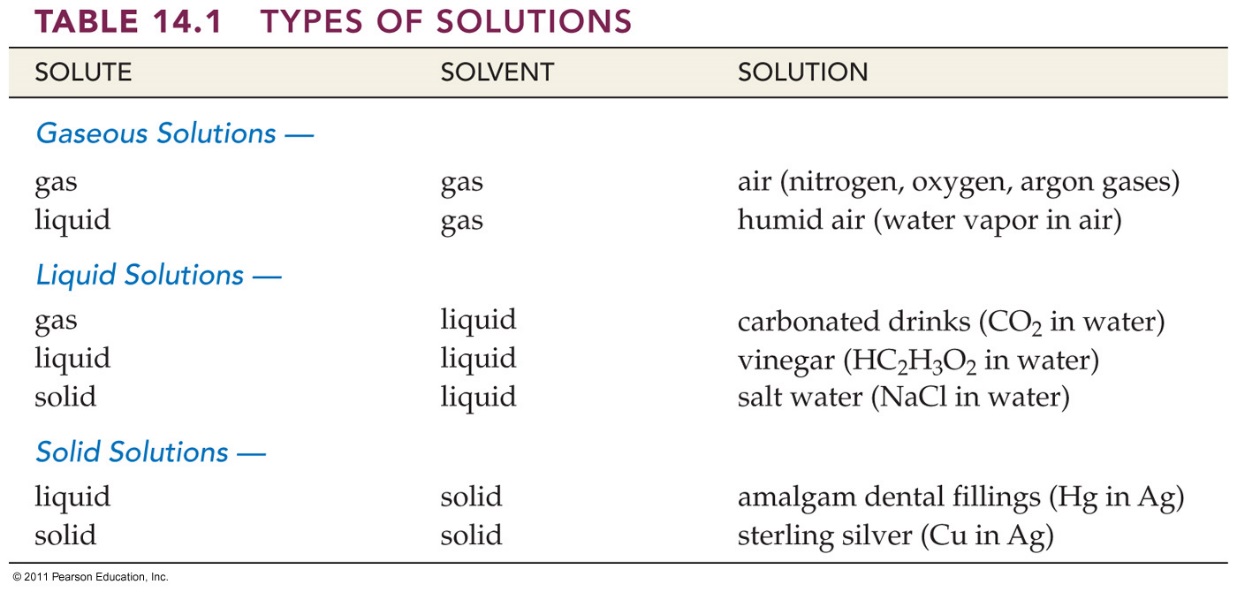
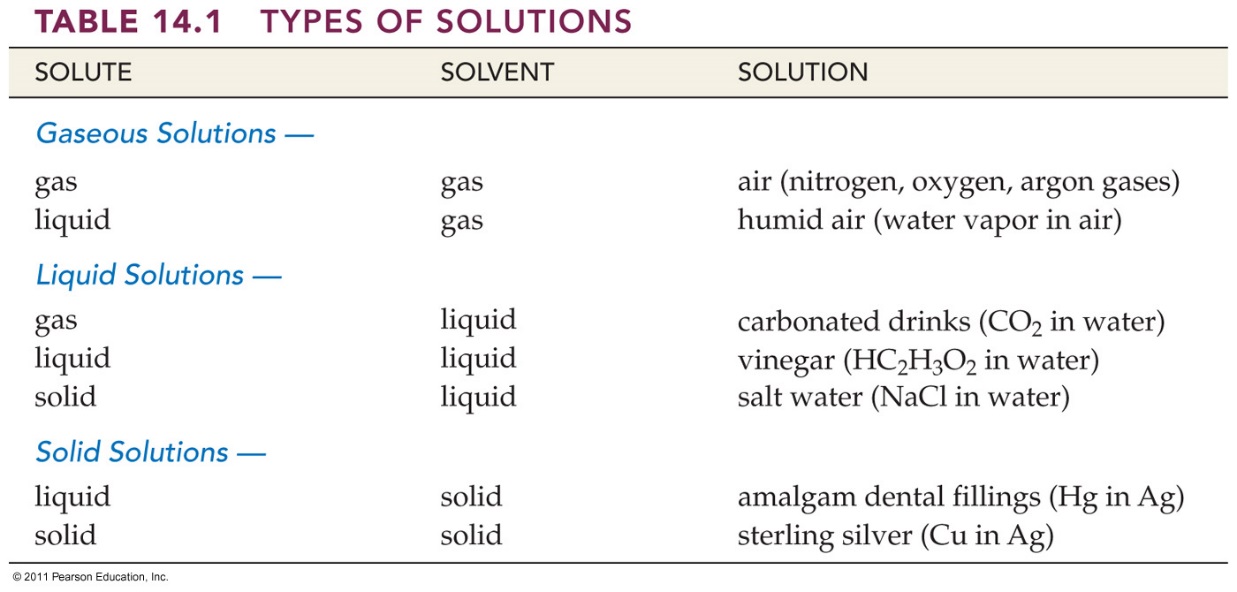
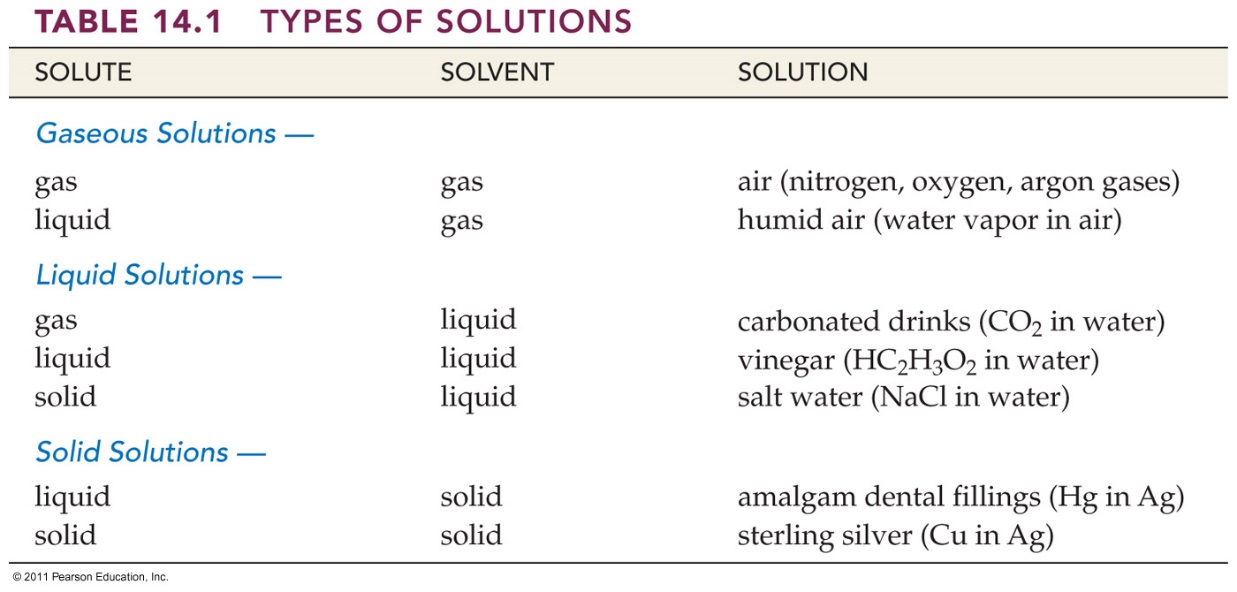
liquids, Table 14.1 Corwin 6th lists some common examples of
solutions whose physical state correspond to that of a solvent.


Chapter 7 starts with six properties of a true solution which are not
listed in chapter 4 as a separate list. You will write five of these six
properties for Part A of Chapter 7 Path 4 Exam:
1. It is
homogeneous mixture of two or more components, solute and
solvent
2. It
has variable composition, that is, the ratio of
solute and solvent may be varied.
3. The dissolved
solute is molecular or ionic in size
4. It may be
colored or colorless but it is usually transparent
5. The solute
remains uniformly distributed throughout the solution
and will not settle out with time (every drop
has exactly the same concentration)
6. The solute
generally can be separated from the solvent by purely
physical means (for example evaporation or distillation)
Part A: Solution Properties
List five of the six properties of a true solution:
(1)
(2)
(3)
(4)
(5)
(6)
Part B: Dissolving
Process






Part B1: Factors
Affecting Rate of Dissolving
Part
B & B1 covers the dissolving process and a discussion of solubility and
temperature plus solubility and pressure of a gas in a solution is as follows.

The
following answers the first question:
State
two factors greatly affecting the solubility of a gas in a liquid:
(1) Temperature (increased temperature of
a solvent also generally increases the kinetic energy of the solute and
the gas solute acquire more of a tendency to escape from the solvent.
Therefore, Cooling the solvent increased the
solubility of a gas in a liquid solvent.)
(2) Pressure (increasing the pressure
(partial pressure) of a gas solute increases the solubility proportionally of
that solute in the liquid (Henry’s Law)
The
properties of liquids dissolved in liquids focus on the main property which is
polarity. Now is the time to review polar covalent bonds chapter 6. Polar covalent bonds depend on the
electronegativities of the two elements.

If two elements
differ in electronegativity between 0.4 and 1.7, then polar covalent bonds are
formed. If the difference between the two atoms is greater than 1.7 then
ionization takes place. A solution containing ions must be dissolved by polar
molecules as a solvent.
Next you must
understand the three dimensional geometry of the
molecules to determine if a molecule is polar. Molecules with polar covalent
bonds have dipoles, which are vectors, created by the polar covalent bonds When
they are summed, if there is a net moment of force the molecule is polar.
However, it is possible for a compound to have polar covalent bonds and be
nonpolar when the net summation of the vectors total zero.
The “Like Dissolves Like Rule” depends on the polarity of the molecules of the
solute and the solvent (Table14.3 Corwin 6th is the same as Table
13.3 on page 378..


What
is the main factor affecting the solubility of a liquid in a liquid:
(3) Nature of the solute and solvent: the like dissolves like rule. The general principle that solubility is greatest when the
polarity of the solute is similar to that of the
solvent

From Corwin’s 6th
edition
The discussion of
the dissolving leads to the rate of dissolving, which is the next question in
Section B1 of Chapter 7
(They do not list #3 below.).

State four factors which governs the
rate of dissolving a solid in a liquid:
1. Particle
Size (increased surface area increases rate of solution i.e
powders have greater surface area than crystals
and will dissolve faster)
2. Temperature (increased
temperature of solvent generally increases rate of solution, except
gases in liquids is opposite)
3. Concentration
of Solution- when the solute and solvent are first
mixed the rate of dissolving is at a maximum, as saturation approaches the rate
of dissolving slows
4. Agitation or
stirring-the effect of agitation is kinetic
which increases the rate of solution.




Part B1: Factors Affecting Rate of Dissolving
State two factors greatly affecting the solubility of a gas in a liquid
and explain:
(1)
(2)
What is the main factor affecting the solubility of a liquid in a liquid
and explain the rule:
(3)
State four factors which governs the Rate of
dissolving a solid in a liquid:
(1)
(2)
(3)
(4)
Part C: Intermolecular Forces






Part C: Intermolecular Forces
1. Describe the different type of interparticle forces that can occur
between atoms, molecules, and ions.
2. Distinguish between the forces called intermolecular forces.
3. What forces are referred to as van der Waals forces?
4. Draw a flow chart or diagram to summarize these intermolecular forces
and show an example.
|
Type of Interaction |
Factors Responsible For Interaction |
Approximate Energy (kJ/mol) |
Example |
|
Ion-dipole |
|
|
|
|
Dipole-dipole |
|
|
|
|
Hydrogen Bonding, X—H…:Y |
|
|
|
|
Dipole-induced dipole |
|
|
|
|
Induced
dipole-induced dipole (London dispersion
forces) |
|
|
|
5.
What are London Forces?
Part D: Concentration of Solutions
General Words:



Specific
Words/Terms of Concentration:




There are three measurements of solutions in preparation
problems of which two will be given and the third will be asked in Part D
for preparing a solution in a laboratory. The three are: mass of solute, volume of
solution (not volume of solvent-you should know the difference), and the
concentration of the solution.
There are six methods of measuring the concentration of a solution: Molarity, Weight (Mass)
Percent, Volume Percent, Molality, Parts Per Million, and
Normality. Problems for Part D will focus mainly on Molarity, but Weight
percent is also fair game. The other four methods of measuring concentration
will not be asked in Part D.
Part
D1: Preparation of Solutions Calculations
If the
problem states the mass of the solute and the volume of the solution prepared
is given, then the Molarity is unknown for one problem type. The other common problem is how to make a known volume of a known
concentration of a solution and you have to find the mass.
1. How many grams of solute are needed to prepare 250 ml of a 0.0100 M KMnO4?
2. 20 grams of AgNO3 were placed in a 250 ml volumetric flask, calculate the Molarity of the Solution.