Project #25 Isomer Number Problem
(Chapter 12)
Isomers of Alkanes,
Alkyl halides, Alkenes, and Cycloalkanes 10 points
After reading chapter 12, isomerism is
not discussed. This project is to look at structural isomerism. There is also
geometric isomers (which is discussed) and optical isomerism in the study of
organic chemistry.
To understand organic molecules you should
have a grasp of molecular structure introduced in Chapter 6. Building
structural isomers will strengthen your knowledge of building molecules.
Usually it is helpful to have a molecular models kit, but you can assemble molecular
structures with tooth picks and gum drops.
What is Structural Isomerism?
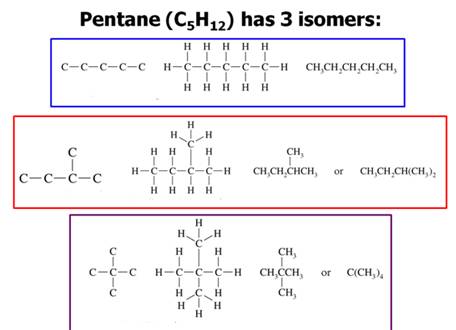
Structural isomerism, (or constitutional isomerism) is a form of isomerism in which molecules with the same molecular formula have bonded together in different orders making different compounds Three categories of structural isomers are skeletal, positional, and functional isomers. Positional isomers are also called regioisomer.
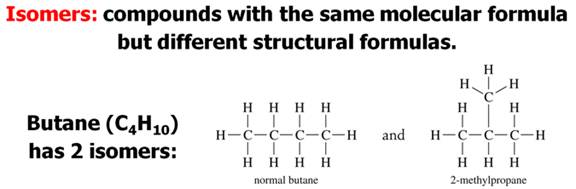
In chain isomerism, or skeletal isomerism, components of the (usually carbon) skeleton are distinctly re-ordered to create different structures.

View the following links for additional help:
Alkane Prefixes: Count to Ten in Organic
Alkane Series
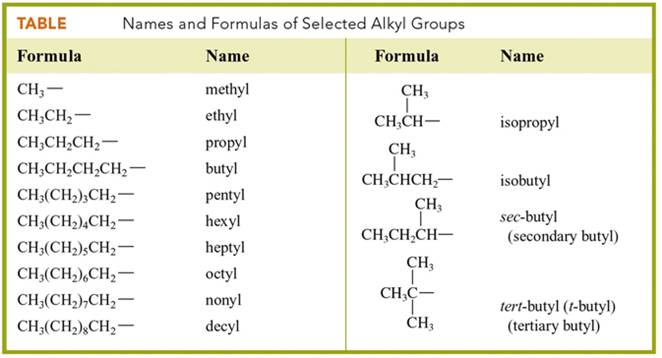
Alkyl
Groups C1-C8
All
Possible Alkyl Groups C1-C4
IUPAC Naming System for Organic Compounds


The following are additional web pages
demonstrating the process to draw different isomers:
Worked Example:
Naming Alkanes
Naming Alkanes-Page 2
Naming Alkanes-Page 3
Naming Alkanes-Page 4
Naming Alkanes-Page 5
Worked
Example: Naming Alkenes
Naming
Alkenes-Page 2
Naming Alkanes-Page 3
Isomer
Number Problems
Three Pentane
Isomers
Writing
Isomers 1
Writing
Isomers 2
Another Worked Example:
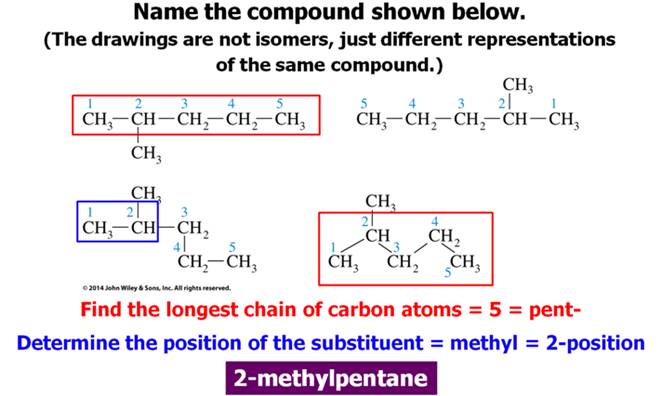
Look at the following two examples:
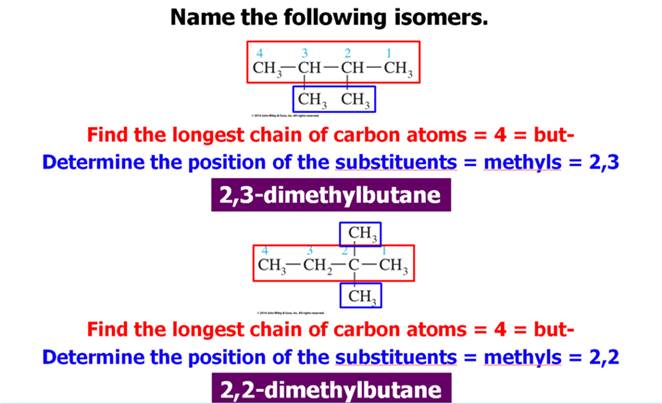
Structural Isomers: Alkanes:
IUPAC
Rules for Alkane Nomenclature
1. Find and name the longest continuous carbon
chain.
2. Identify and name groups attached to this chain.
3. Number the chain consecutively, starting at the end
nearest a substituent group.
4. Designate the location of each substituent group by an
appropriate number and name.
5. Assemble the name, listing groups in alphabetical order
using the full name (e.g. cyclopropyl before isobutyl).
The prefixes di, tri, tetra etc., used
to designate several groups of the same kind, are not considered when
alphabetizing.
Sample Video for drawing Structural isomers of alkanes:
http:/www.fscj.me/chm1020/Projects/Project25IsoerNumberProblems/Hexane_Isomers.mp4
Structural Isomers: Alkyl halides:
The halogen is treated as a substituent on an alkane
chain. The halo- substituent is considered of equal rank with an alkyl
substituent in the numbering of the parent chain. The halogens are represented
as follows:
|
F |
fluoro- |
|
Cl |
chloro- |
|
Br |
bromo- |
|
I |
iodo- |
Here are some
examples:
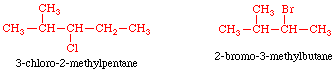
Worked Example: C4H9Br
4 isomers (Bromobutanes);
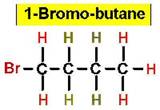
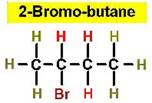
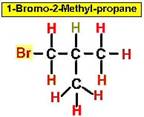
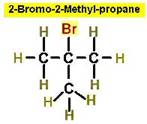
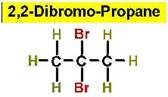
Worked Example: C3H6Br2
4 isomers (Dibromopropanes);
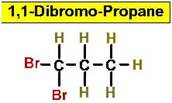
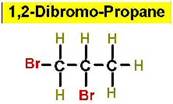
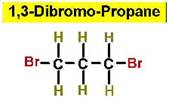
Structural Isomers: Cycloalkanes
Cycloalkanes have one or more rings of carbon atoms.
The simplest examples of this class consist of a single, unsubstituted
carbon ring, and these form a homologous series similar to the unbranched alkanes. The IUPAC
names of the first five members of this series are given in the following
table. The last (yellow shaded) column gives the general formula for a cycloalkane of any size. If a simple unbranched
alkane is converted to a cycloalkane
two hydrogen atoms, one from each end of the chain,
must be lost. Hence the general formula for a cycloalkane
composed of n carbons is CnH2n. Although a cycloalkane has two fewer hydrogens
than the equivalent alkane, each carbon is bonded to
four other atoms so such compounds are still considered to be saturated
with hydrogen.
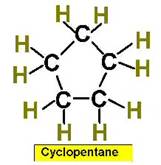
Examples of Simple Cycloalkanes
|
||||||
|
Name |
Cyclopropane |
Cyclobutane |
Cyclopentane |
Cyclohexane |
Cycloheptane |
Cycloalkane |
|
Molecular |
C3H6 |
C4H8 |
C5H10 |
C6H12 |
C7H14 |
CnH2n |
|
Structural |
|
|
|
|
|
(CH2)n |
|
Line |
|
|
|
|
|
|
Substituted cycloalkanes are named in a fashion very similar to that
used for naming branched alkanes. The chief
difference in the rules and procedures occurs in the numbering system. Since
all the carbons of a ring are equivalent (a ring has no ends like a chain
does), the numbering starts at a substituted ring atom.
IUPAC
Rules for Cycloalkane Nomenclature
1. For a monosubstituted
cycloalkane the ring supplies the root name (table
above) and the substituent group is named as usual. A location number is
unnecessary. |
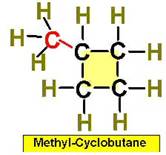
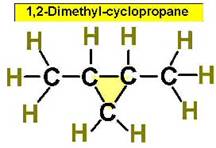
Worked Examples: C5H10 5 isomers (cycloalkanes only)
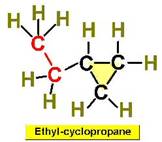
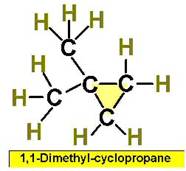
Structural Isomers: Alkenes
IUPAC
Rules for Alkene and Cycloalkene
Nomenclature
1. The ene suffix (ending) indicates
an alkene or cycloalkene.
2. The longest chain
chosen for the root name must include both carbon atoms of the double bond.
3. The root chain must be numbered from
the end nearest a double bond carbon atom. If the double bond is in the center of the chain, the nearest
substituent rule is used to determine the end where numbering starts.
4. The smaller of the two numbers designating the carbon
atoms of the double bond is used as the double bond locator. If more than one
double bond is present the compound is named as a diene,
triene or equivalent prefix indicating the number of
double bonds, and each double bond is assigned a
locator number.
5. In cycloalkenes the double
bond carbons are assigned ring locations #1 and #2. Which of the two is #1 may
be determined by the nearest substituent rule.
6. Substituent groups containing double bonds are:
H2C=CH– Vinyl group
H2C=CH–CH2– Allyl
group
Worked Examples: C5H10 5 isomers (alkenes only)
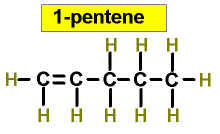
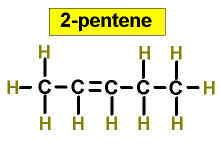
(There is no 3-Pentene)
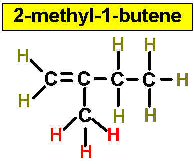
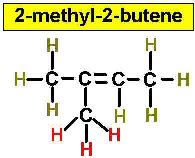
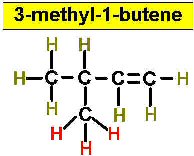
Project #25 Assignment:
Draw the structural or semi-structural formulas for
all the isomers of the following chemical formulas listed below, then give the IUPAC name for each:
You may do it
on separate paper or fill out the following Isomer # Report Form:
http:/www.fscj.me/chm1020/Projects/Project25IsoerNumberProblems/Project25IsomerReportForm.htm
Submit hard
copy by the last class meeting, or submit electronic copy the Last day of the
term.
#1 Alkane: C6H14
5 isomers - 1 point
Watch
Video:
http:/www.fscj.me/chm1020/Projects/Project25IsoerNumberProblems/Hexane_Isomers.mp4
#2 Alkane C7H16
9 isomers - 2 points
#3 Alkyl Halide: C5H11Br 8 isomers
- 2 points (See Example C4H9Br above)
#4 Alky Halide (Dienes) C3H6Br2
9 isomers - 2 points (See Example C4H9Br
above)
#5 Cycloalkanes C6H12 12 isomers (cycloalkanes
only) - 2 points (See Example C5H10 above)
#6 C6H12
Alkenes 13 isomers (alkenes only) - 2
points (See
Example C5H10 above)
#7 C4H10O
Alcohols and Ethers 7 isomers (Alcohols & Ethers) – 1 point
(Answer
Links made available after Homework is submitted)