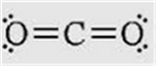Projections of the data Collected/Calculated



The last
part of the project is for you to do the annual projections and summary of CO2
emissions. This is worth 10 points.
- What
is your annual mileage?
Annual Mileage = your
daily average miles driven calculated above multiplied by 365 days in a year
- What
is your projected annual need for gasoline?
Annual Gasoline Demand = Your average daily Gallons Used multiplied by 365 days in a year
- What would be your annual cost at $2.00 per gallon;
$3.00 per gallon; $3.50 per gallon; $4.00 per gallon; $4.50 per gallon;
and $5.00 per gallon.
Annual Cost @ $2.00/gallon = Yearly Gasoline Demand(gallons) multiplied by $2.00/gallon
Annual Cost @ $3.00/gallon = Yearly Gasoline Demand(gallons) multiplied by $3.00/gallon
Annual Cost @ $3.50/gallon = Yearly Gasoline Demand(gallons) multiplied by $3.50/gallon
Annual Cost @ $4.00/gallon = Yearly Gasoline Demand(gallons) multiplied by $4.00/gallon
Annual Cost @ $4.50/gallon = Yearly Gasoline Demand(gallons) multiplied by $4.50/gallon
Annual Cost @ $5.00/gallon = Yearly Gasoline Demand(gallons) multiplied by $5.00/gallon
- Total Pounds
of Carbon Dioxide released into the atmosphere by you every year.
Total Annual CO2 Released = Total
Annual Gallons Used multiplied by 18.7 pounds/gallon
This experiment is equivalent
to four small projects (two wet chemistry labs) as it is an intense A-15/B-12: 10-14 week exercise in data collection
or
an intense A-7: 5-7 weeks exercise in data collection
for 20-60 points depending on the number of weeks data is collected.
(If you have two cars, you may
do two projects. The second car will earn extra credit. Then you may compare
the efficiency or lack of for your fleet of autos. If you change cars during
the project, you have to make some estimations-talk
with your instructor how to switch cars during the project and maintain the
accuracy of the project Although it will be interesting to see if there is a
difference between the two vehicles, it still could as only one project. Maybe
a little extra credit for presentation.
The chemical reaction for combusting
gasoline is:
2 C8H18
(l) + 25 O2 (g)
à 16
CO2 (g) + 18 H2O (g)
Octane
burns in oxygen gas to form carbon dioxide and water as products ,which
comes out your tailpipe
|
|
+ |
|
à |
|
+ |
|
(Burn 1
gallon put 18.7 Lbs CO2 in the
environment!)
In CHM 1025C & CHM 1020 Chapter 9 introduces
Mass Stoichiometry.
(In CHM 2045C Chapter 3 begin
stoichiometry)
We will prove the 18.7 lb
CO2/gallon statement when we study chemical reactions and mass
stoichiometry in Chapter 9.
Do not worry about this Calculation until we get to chapter 9 Section 9.2
Show a dimensional analysis setup in your
project to prove this in the conclusion of your project.
Unit Factors Needed: 3.79L = 1 Gal 0.680g C8H18 = 1L 453.56g = 1 lb 1 L = 1000 mL
2.205lb = 1kg 1000g = 1kg 114gC8H18 = 1 moleC8H18 44.0g CO2 = 1 mole CO2
*You need to only fill the tank twice, at the beginning and at the end of the
project. You will not use the first fill-up in your calculations, except
odometer reading. Why?
The instructor may add
additional data for you to determine to complete this project after studying chapter
9.
If you do not drive or own a vehicle
and can not get cooperation from your family, the
instructor will be assigned an alternate energy demand project (My Electric
Demand!) or you may earn partial credit using the data of your instructor’s car
(4 years instead of 2-5 months).
Project Conclusion/Summary
Write a summary/Conclusions and statement of what you learned from this project
(at least one paragraph). In your
Summary paper, the data above and below states we are doing better conserving
our gasoline while more cars are on the road and the price for the last year or
two has dropped 50%! Why? (include a paragraph)
In your project conclusion describe what the octane
rating means and what octane you use in your vehicle.
Research
the Internet. And see if you can find additional information.
I found the following:
In 2015 the EIA
(United State Energy Administration) reported:
How much gasoline does the United States
consume?
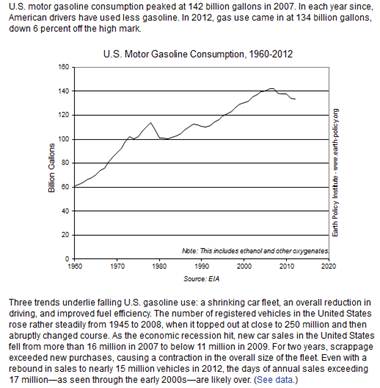
In 2015, about 140.43 billion
gallons (or about 3.34 billion barrels1) of gasoline were consumed2
in the United States, a daily average of about 384.74 million gallons (or about
9.16 million barrels per day).3 This was about 1.5% less than the
record high of about 390 million gallons per day (or
about 9.29 million barrels per day) consumed in 2007.
1
There
are 42 U.S. gallons in a barrel.
2 EIA uses product supplied to represent approximate
consumption of petroleum products. Product supplied measures the disappearance
of these products from primary sources, such as refineries, natural gas
processing plants, blending plants, pipelines, and bulk terminals.
3 Preliminary data for 2015.
We Are Using Less Gasoline Today
U.S. Total Gasoline Retail Sales
by Refiners (Thousand Gallons per Day)
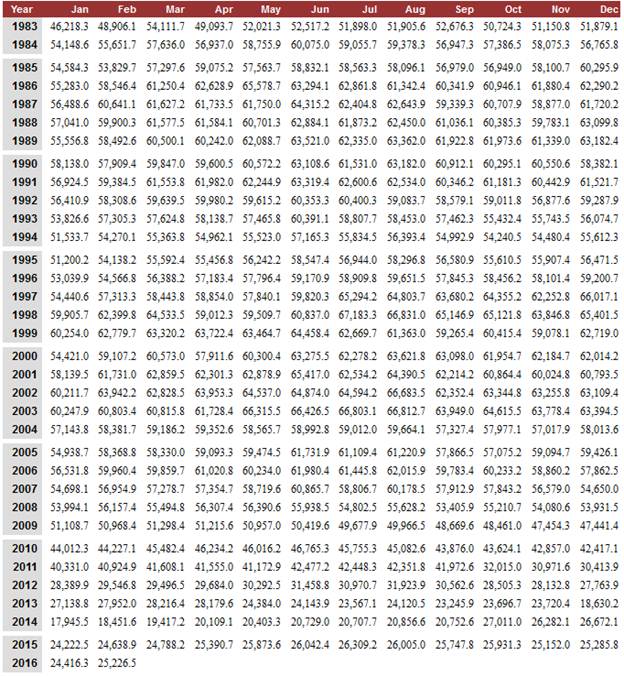
Update of Gasoline Usage in the
United States
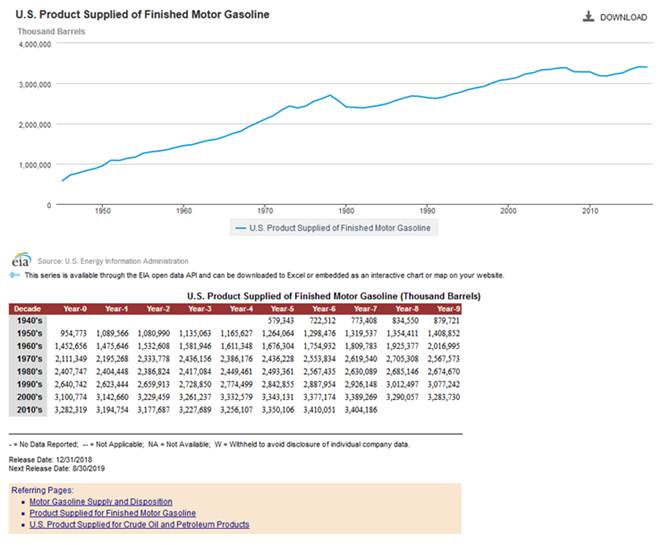
URL: https://www.eia.gov/dnav/pet/hist/LeafHandler.ashx?n=PET&s=MGFUPUS1&f=A
Average U.S. gasoline usage lowest in 3 decades, study says (Automotive News March 2015)
With improvements in vehicle fuel economy, U.S. drivers’ average gasoline
consumption is the lowest it’s been in at least 30 years, according to research
by the University of Michigan released today.
The number of gallons of gasoline
used per person, driver, vehicle and household is below rates in 1984, when the
study was first conducted, according to researcher Michael Sivak
of the University of Michigan Transportation Research Institute.
In 2013, gallons of gasoline
consumed per person (392) fell 17 percent from 2004, gallons used per driver
(583) fell 16 percent, and gallons used per household (1,011) fell 19 percent.
2004 was the year of maximum consumption for those categories.
URL: https://www.eia.gov/dnav/pet/pet_cons_psup_a_EPM0F_VPP_mbbl_a.htm
Article Continues:
Gallons used per vehicle (524)
dipped 14 percent from 2003, which was its maximum consumption year.
Even though population grew 8
percent from 2004 to 2013, total fuel consumed by light vehicles decreased 11
percent, Sivak said in a statement.
In 1984, annual fuel consumption
rates were slightly higher than in 2013: 400 gallons per person, 608 gallons
per driver, 602 gallons per vehicle and 1,106 gallons per household.
The study also found that the number
of vehicles and distance driven per person, driver, vehicle and household are
at their lowest since the 1990s, the statement said.
The declining number is driven not
only by economic factors, but also rises in telecommuting and use of public
transportation, Sivak said.
“The reductions in the
fuel-consumption rates reflect, in part, the added contribution of the
improvements in vehicle fuel economy,” he said in a news release.
“Per person, per driver and per
household -- we now have fewer light-duty vehicles, we drive each of them less
and we consume less fuel than in the past,” Sivak
added.
Sivak and fellow researcher Brandon Schoettle
also compile an average fuel economy report each month.
Why
do we not add the # gallons and Total Spent
on the initial fill-up in the Project Totals at the
bottom of your data presentation?
How
do we determine the #days in the project?
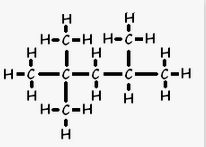
One of the Octane Molecules we will
study in Chapter 12
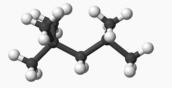 2,2,4 Trimethylpentane
2,2,4 Trimethylpentane
What does this octane rating mean?
In your project conclusion describe what the octane rating means and
what octane you use in your vehicle.
Premium: 91-93 Octane Midgrade: 89 octane Regular: 87 octane
Can you buy 100 octane gasoline?