207th 2YC3 Conference Abstract
Origin of the Elements
Dr. Mike Reynolds; Dr Joseph
Langat; and Professor John Taylor
Florida State College @ Jacksonville

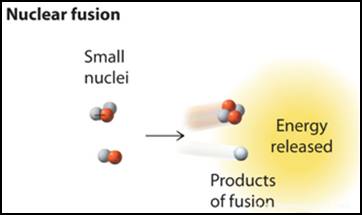
Thermonuclear Fusion Nuclear Change: Nuclear Fusion
When we teach a chapter on Thermodynamics, the Standard Enthalpy
of Formation of an element is defined as zero joules/mole.
Have you every placed the question to the students:
Why?
Dr. Joseph Langat has been using clickers to
stimulate students in his chemistry classrooms to attempt them to “Think
Critically”
After a few ideas are proposed between students, show a video clip from the
2010 Science/Discovery Channel Series: How
the Universe Works - Season 1: Episode #3: The Big Bang Theory or a clip
from National Geographic’s Naked Science Series “Birth of the Universe”.



One segment discusses the creation of matter and then antimatter and a minute
imbalance one unit in 100,000,000 crates matter from energy, then an electron, then
a proton, then the element hydrogen. This clip will be shown to the
participants.
Nuclear Fusion created the elements from helium to iron with an incredible
amount of energy needed, but higher masses of elements could not be formed in
the beginning until another event happened!
Traditionally texts do not delve into Nuclear Chemistry until the end of the
book. Most do not discuss the origin of the elements, until recently Wiley’s
Jespersen’s 7tgh edition has two pages summarizing the two above films in
Chapter Zero.
Now discussions of radiation and nuclear change are introduced early in these
texts, before thermodynamics. To end the lesson, again the students are poled
about the original question: Why?
Go a step farther ask the students how old are the atoms of oxygen, carbon,
nitrogen, and hydrogen in their bodies that are part of our life cycle?