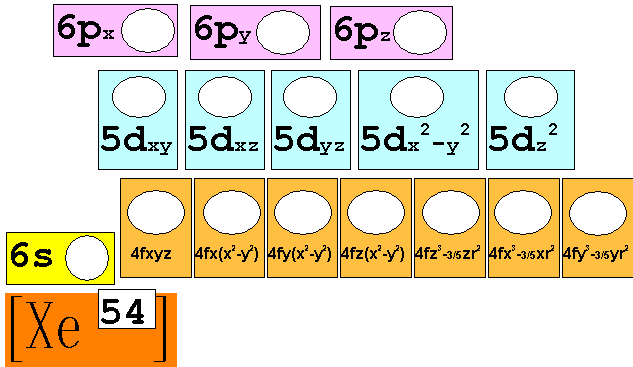CHM
1025C†††††††††††††††† †††††††††††††††††††††††† Name:
__________________
†††††††††††††† Module Three Homework Packet-Hein
Module 3i: Atomic Theory &
The Periodic Chart (Chapter 5 ,10)
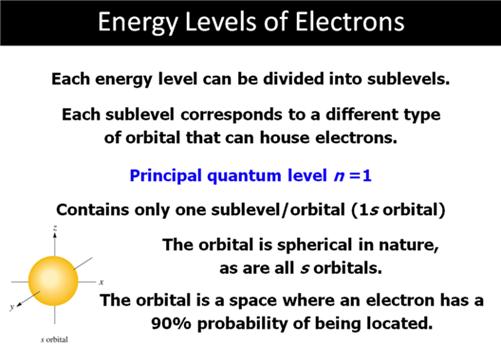
A. _____ (02) Atomic Notation-Section
5.5 Answers
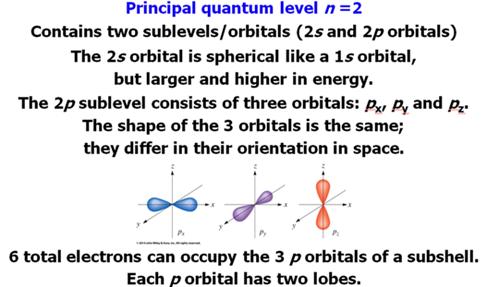
B. _____ (08) Electron Configuration-Sections
10.4 Answers
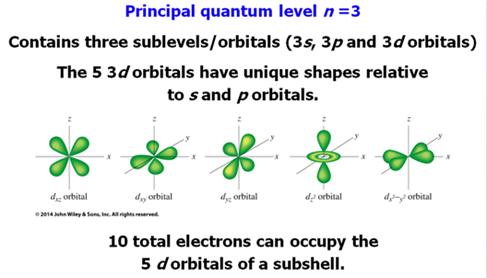
C. _____ (03) Orbitals
/ Subshells of the Periodic Table-Section 10.5 Answer
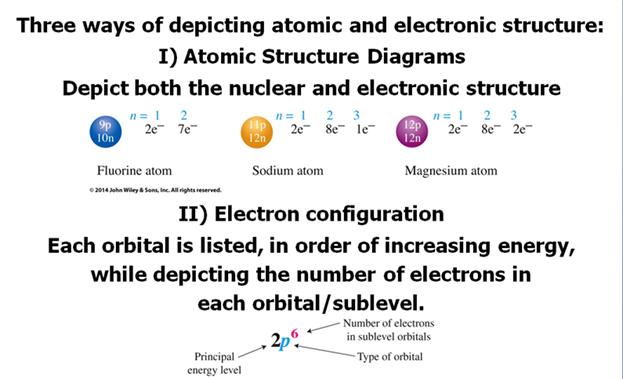
C1. ____ (06) Spectroscopic
Notation from Periodic Chart Section 10.5† Answer
_______(19) Module 3i Total (Fifth Exam)
Module 3ii: Atomic
Theory & The Periodic Chart (Chapter 5, 6, & 11)
D. _____ (02) Electron Dot
Structures-Section†
11.2 Answers
E. _____ (03) e-1
Configuration of Ions-lecture† Answers††††††††††††††††††††††††††††††
F. _____ (02) Periodic Ionic
Character-Section 6.2 Answers
P. _____ (04) Periodic Chart
Identifications Ė Chapter 5 Answer
_______(11) Module 3ii Total
(Sixth Exam)
Module Three: Part A†† Atomic Notation††††††††††††††††††††††† 1 point
Given the following
elements, atomic numbers, and mass numbers, State the number of electrons,
protons, and neutrons in the following elements:
1.†††† 23Na11†††††††††††††††††††††††††††† Protons††† = ______
Electrons = ______
Neutrons† = ______
2.†††† 93Nb41†††††††††††††††††††††††††††† Protons††† = ______
Electrons = ______
Neutrons† = ______
3.†††† 20Ne10††††††††††††††††††††††††††† †Protons†††
= ______
Electrons = ______
Neutrons† = ______
4.†††† 59Ni28††††††††††††††††††††††††††††† Protons††† = ______
Electrons = ______
Neutrons† = ______
5.†††† 19F9†††††††††††††††††††††††††††††††† Protons††† = ______
Electrons = ______
Neutrons† = ______
Additional Homework
(not required) for your practice:
Corwin 7th
edition Section 4.4: Page 127 Questions #17-24
Hein 14th
edition Section 5.5: Page 05 Questions #9-26 especially #17-18
Module
Three: Part B:†† Electron
Configuration††† 5 points
Given the following
elements and atomic numbers, use arrows to fill-in the electron configuration,
then rewrite the configuration into the chemistís shorthand (spectroscopic
Notation):
*remember 4/9 rule
exceptions: Never 4/9 total d orbital electrons except for elements 41, 74, 78.
1.† 12C6††††††††
Chemist Shorthand:_____________________________________
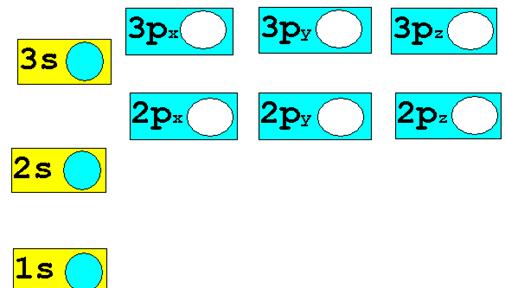
2. 60Co27††
Chemist Shorthand:_____________________________________
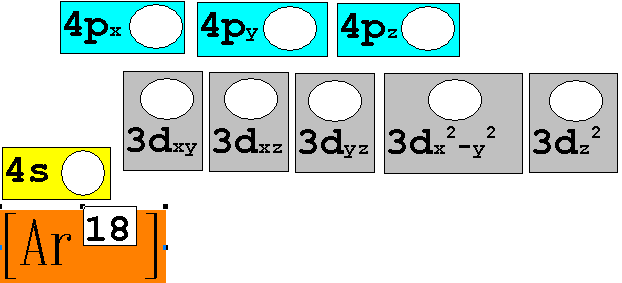
Module
Three: Part B: Electron Configuration continued:
3.† 40Ca20†††††† ††Chemist Shorthand:____________________________

4.† 52Cr24†††††††† Chemist Shorthand:____________________________

Module
Three: Part B: Electron Configuration continued:
5.† 65Zn30†††††††† Chemist Shorthand:____________________________

6.† 85Nb41†††††††† Chemist Shorthand:____________________________
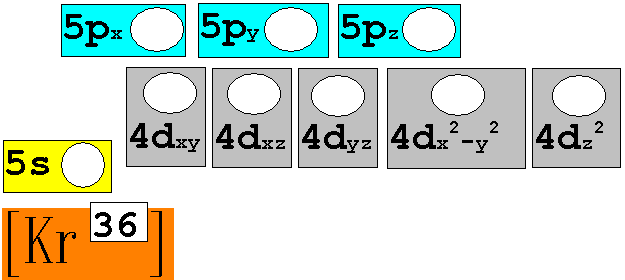
Module
Three: Part B: Electron Configuration continued:
7.† 96Mo42†††††††† Chemist Shorthand:____________________________

8.† 180Au79†††††††† Chemist Shorthand:____________________________
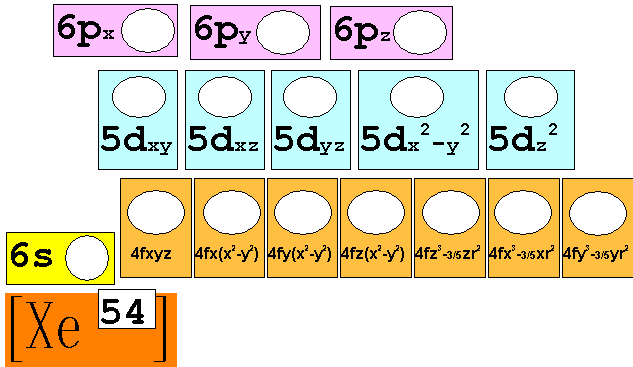
9.
Pd46†††††††† Chemist
Shorthand:____________________________

10.† Pt78†††††††† Chemist Shorthand:____________________________
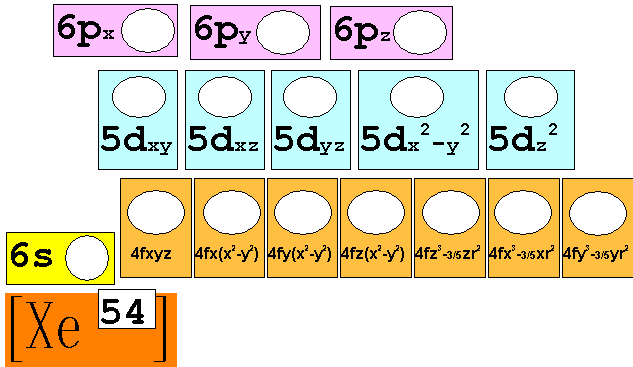
11.† Ag47†††††††† Chemist Shorthand:____________________________

12.† W74†††††††† Chemist Shorthand:____________________________
Interactive Electron Configuration
Web Site:
http://www.lsua.info/e_config/e-1instruct.html
Electron Configuration Rules Menu:
http://www.fccj.info/e_configMenu/e-1Menu.html

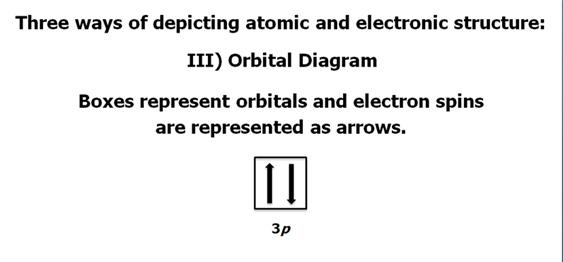
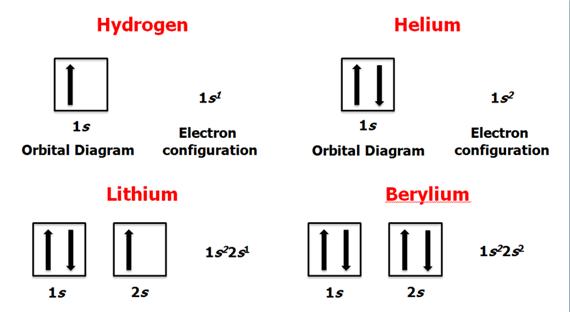
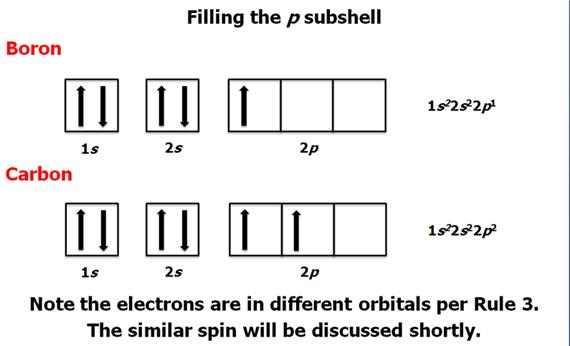

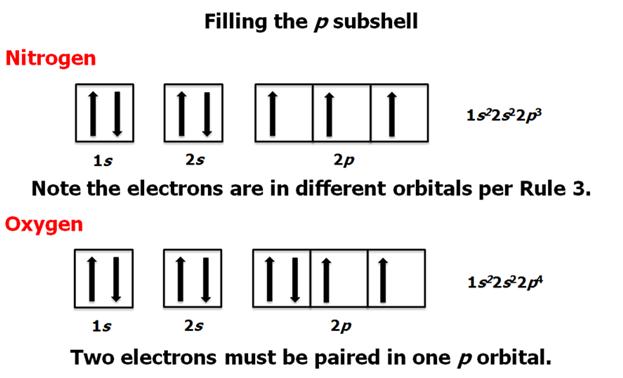
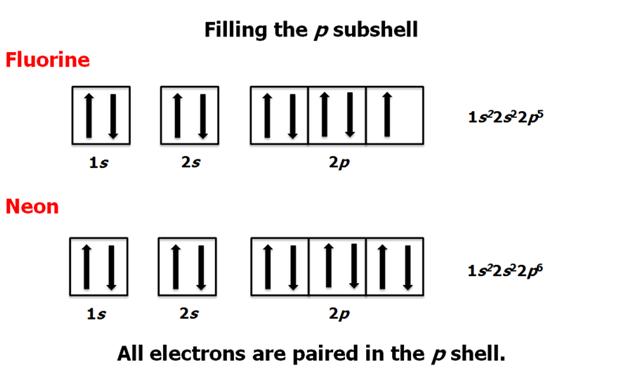
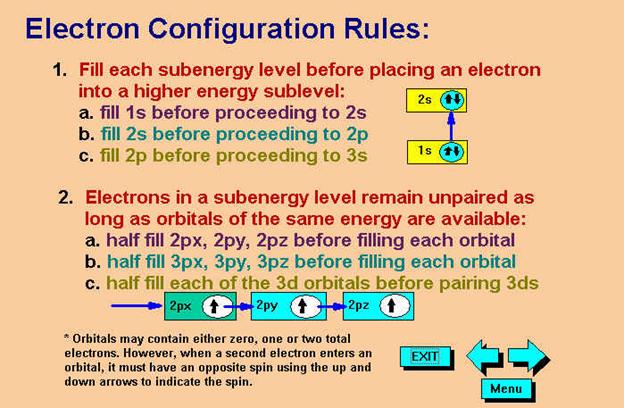

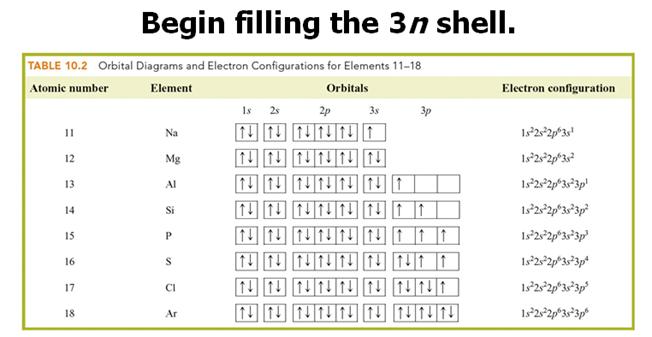
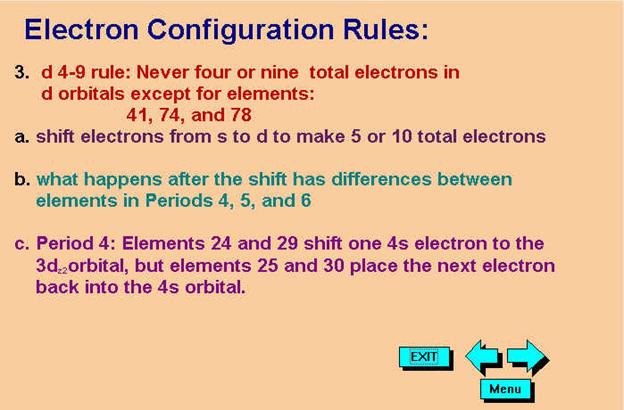
Check
Your Answers. Click on the element on the periodic table:
http://fscj.me/e-1Spectroscopic/pc.html
Animation
of Elements 1-112 filling electrons:
http://www.northcampus.net/ElectronConfiguration/SpectroscopicNotation/spectroscopicNotation.html
Additional reference
for your information
Corwin 7th
Sections 4.10-4.11 Pages 119-129
Hein 14th
Sections 10.3-10.4 Pages 195-201
Additional Homework
(Not required) for your practice and study for MC exam:
Corwin 7th:
Energy levels and Subshells: Questions #65-72
Corwin 7th:
Electron Configuration: Questions #73-78
Corwin 7th:Quantum Mechanical Model of the Atom: #79-86
Hein 14th: Orbitals/e1-
Configuration Questions #7-30 especially #11-22
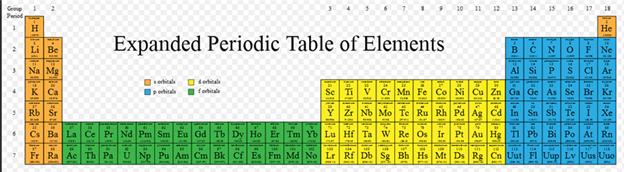
Module
Three: Part C Orbital Subshells & Periodic
Chart†† 1 point
On
the periodic chart below show all the s, p, d and f block elements on the the first six rows of the periodic table (Label each area
beginning with 1s, 2s, 2p, etc):
|
periodic
table
|
|
Group
|
1
|
2
|
|
3
|
4
|
5
|
6
|
7
|
8
|
9
|
10
|
11
|
12
|
13
|
14
|
15
|
16
|
17
|
18
|
|
Period
|
|
|
1
|
1
†
|
|
2
†
|
|
2
|
3
†
|
4
†
|
|
5
†
|
6
†
|
7
†
|
8
†
|
9
†
|
10
†
|
|
3
|
11
†
|
12
†
|
|
13
†
|
14
†
|
15
†
|
16
†
|
17
†
|
18
†
|
|
4
|
19
†
|
20
†
|
|
21
†
|
22
†
|
23
†
|
24
†
|
25
†
|
26
†
|
27
†
|
28
†
|
29
†
|
30
†
|
31
†
|
32
†
|
33
†
|
34
†
|
35
†
|
36
†
|
|
5
|
37
†
|
38
†
|
|
39
†
|
40
†
|
41
†
|
42
†
|
43
†
|
44
†
|
45
†
|
46
†
|
47
†
|
48
†
|
49
†
|
50
†
|
51
†
|
52
|
53
†
|
54
†
|
|
6
|
55
†
|
56
†
|
*
|
71
†
|
72
†
|
73
†
|
74
†
|
75
†
|
76
†
|
77
†
|
78
†
|
79
†
|
80
†
|
81
†
|
82
†
|
83
†
|
84
†
|
85
†
|
86
†
|
|
7
|
87
Fr
|
88
Ra
|
**
|
103
Lr
|
104
Rf
|
105
Db
|
106
Sg
|
107
Bh
|
108
Hs
|
109
Mt
|
110
Ds
|
111
Rg
|
112
Uub
|
113
Uut
|
114
Uuq
|
115
Uup
|
116
Uuh
|
117
Uus
|
118
Uuo
|
|
|
|
|
*Lanthanoids
|
*
|
57
†
|
58
†
|
59
†
|
60
†
|
61
†
|
62
†
|
63
†
|
64
†
|
65
†
|
66
†
|
67
†
|
68
†
|
69
†
|
70
†
|
|
|
|
**Actinoids
|
**
|
89
Ac
|
90
Th
|
91
Pa
|
92
U
|
93
Np
|
94
Pu
|
95
Am
|
96
Cm
|
97
Bk
|
98
Cf
|
99
Es
|
100
Fm
|
101
Md
|
102
No
|
|
|
Additional reference
for your information
Corwinís 7th
edition: Sections 5.6 †See Figure 5.6 on Pages 143-144
Heinís 14th
edition: Section 10.5 See Figure 10.16 page 204
A similar
Figure:
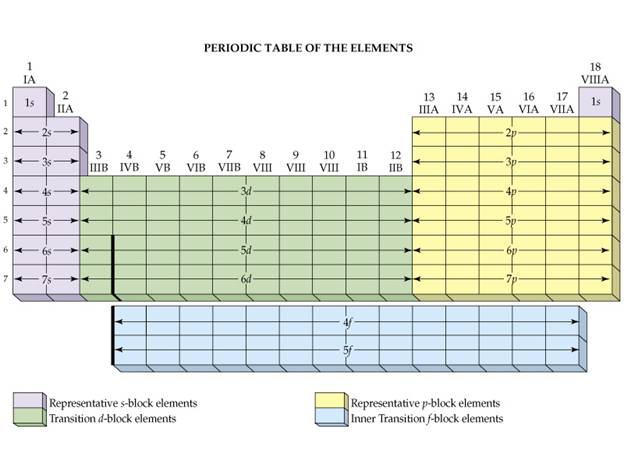
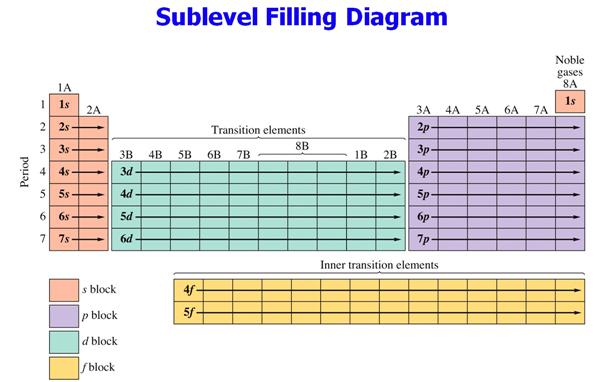
Hein 7th† Page
204
††††††††
Spectroscopic Notation from Periodic Chart
|
1
|
IA
|
IIA
|
|
|
|
|
H
|
|
|
|
|
|
IIIA
|
IVA
|
VA
|
VIA
|
VIIA
|
He
|
|
2
|
|
|
|
|
|
† *
|
|
|
|
|
*
|
|
|
|
|
†
|
†
|
Ne
|
|
3
|
|
|
IIIB
|
IVB
|
VB
|
VIB
|
VIIB
|
VIIIB
|
|
|
IB
|
IIB
|
|
|
|
|
|
Ar
|
|
4
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Kr
|
|
5
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Xe
|
|
6
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rn
|
|
7
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
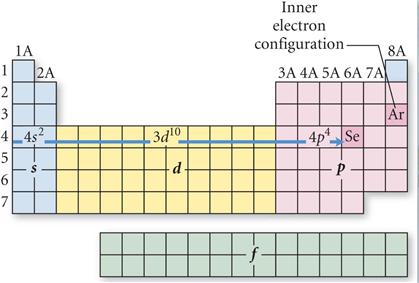
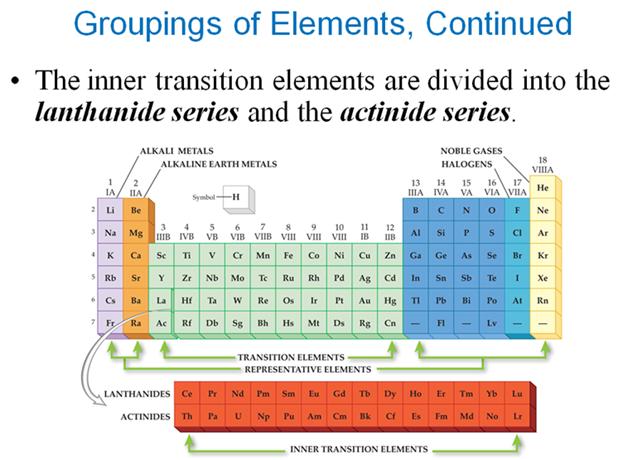
M-3 C1: Spectroscopic Notation
using the Periodic Chart†
2 †Points
Given
the Elementís Atomic Number, use the Periodic Chart above to write the
Spectroscopic Notation for the following elements..
You may do it the long way showing all blocks of orbitals, or you may use the
shorter method applying the square brackets around the Nobel Gas which
indicates the complete inner filled electrons in the core (or Kernal).
i.e:† [Ar]
represents† 1s2 2s2 2p6 3s2
3p6 or
the 18 electrons in the Argon core.
*
In columns VIB and IB, you may have to apply the d4/9 Rule (Never 4/9 total d orbital electrons in any spectroscopic notation
except Nb 41; W 74; and Pt 78)
- 1H††††††††
__________________________________________
- 30Zn†††††
__________________________________________
- 35Br††††††
__________________________________________
- 74W†††† †††___________________________________________
- 8O††††††††
___________________________________________
- 15P†††††††
___________________________________________
- 47Ag††††††
__________________________________________
- 24Cr††††††† ___________________________________________
- 7N††††††††
___________________________________________
- 17Cl†††††††
___________________________________________
Look at the Periodic Table and
Count the squares Left to Right:
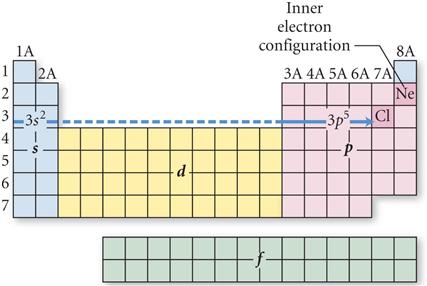
On the fourth row of the
periodic table you have to included the 3d orbitals:
Additional reference
for your information
Corwin 7th:
Section 5.6 Page 144 See example 5.7 p144
Hein
14th: Section 10.5 Pages 201-206 see example10.5 p205/try 10.6
Additional Homework
(Not required) for your practice and study for MC exam:
Corwin 7th : Blocks of Elements: p157 #47-56
Hein 14th : Page 209 Questions 45-46

Module
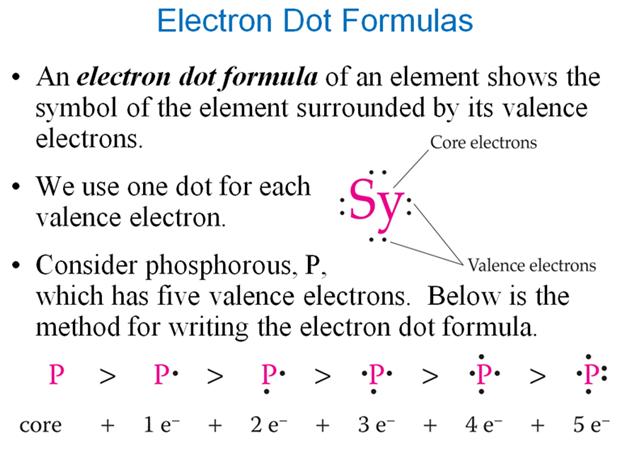
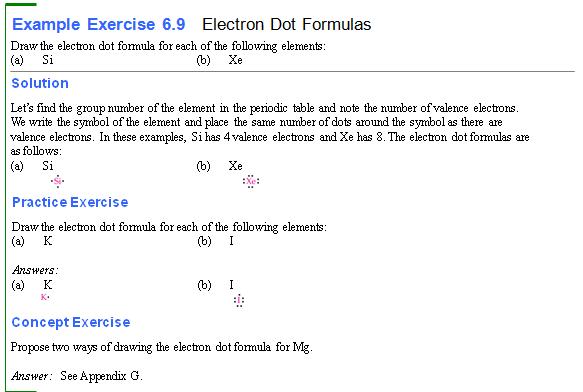
Three: Part D†††††† †Electron Dot Formulas†††††††† 1 point
Using the periodic
chart, draw the electron dot formulas of the following elements (the numbers
shown are the elementís atomic number and mass number):
1.† 6C12†††††††††††††††††††††††††††††††††††††††††††† 6.†† 1H1
2.† 14Si28†††††††††††††††††††††††††††††††††††††††††† 7.†† 7N14
3.†† 9F19†††††††††††††††††††††††††††††††††††††††††††† 8.†† 8O16
4.† 11Na23††††††††††††††††††††††††††††††††††††††††† 9.†† 10Ne20
5.† 15P31††††††††††††††††††††††††††††††††††††††††††† 10.† 16S32
Additional reference
for your information
Corwin 7th:
Section 5.8 Pages 146-147 See example 5.9 p147
Heinís
14th: Section 11.2 Pages 216-217 see figure 11.4 try example 11.2
Heinís
14th: Try Questions 14-15 page 241
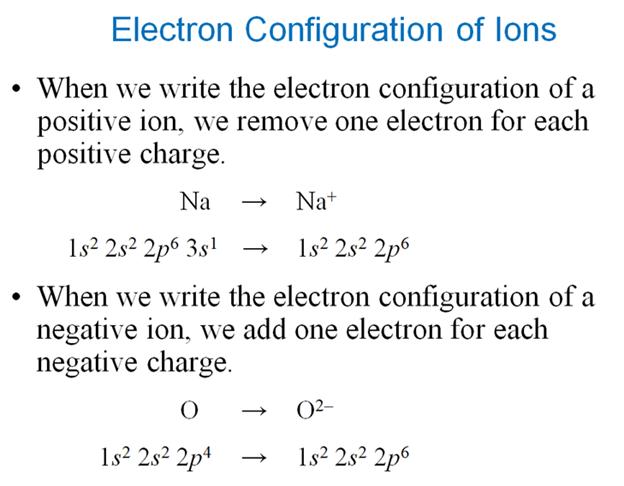
Module
Three: Part E:†† Electron Configuration
of Ions††† 1 †point
Given the following
ions, use arrows to fill-in the electron configuration of the ion, then rewrite
the configuration into the chemistís shorthand:
1.†††† Cl1-† ion††††† Chemist Shorthand:
___________________________

2.† K1+ ion† Chemist Shorthand: _____________________________

Additional reference
for your information
Corwinís 7th : Section 5.10 Pages 151 See Example 5.13
p151-152
Heinís
14th : Section 11.3 Pages217-218† See images page 218
Additional
Homework Problems (not Required) Corwin Ionic Charges P157-8 #75-80
Heinís
14th :Questions #23-24 page 242
Remember
positive ions have lost electrons from the neutral atom, while negative ions
have gained electrons into the neutral atom.
.

Module
Three: Part F†††††† Periodic Ionic
Properties†††††† †1 point
Using
a periodic chart, write the ionic character (monoatomic
ionic charge) of the following elements: (The number before the element is its atomic
number)
1.† 19 K†††† ________†††††††††††††††††††††† 6.††† 9F††††† _____
2.† 20Ca††† _______†††††††††††††††††††††††† 7.††† 1H††††† _____††
†_____††
3.† 7N††††††† _______†††††††††††††††††††††††† 8.††† 16S†††† _____
†
4.† 17Cl††††† _______††††††††††††††††††††††† 9.††† 10Ne†† _____
5.† 53I ††††††† ______†††††††††††††††† ††††††† 10.††
15P†††† _____
Additional reference
for your information
Corwinís 7th:
Section 5.7 Pages 145-146 See example 5.8 p145
Corwinís 7th:
Section 5.10 Pages 149-151 See Examples 5.11-5.12 p150
Heinís
14th: Section 6.2 pages 100-103 See Figure 6.2 page103
Heinís
14th: Section 11.3 Pages 217-222 See Example 11.7 & 11.8 p222
Additional
Homework Problems (not Required)
Corwinís 7th† Ionic Charges P157 #71-74
Heinís 14th p242 Questions #13-16
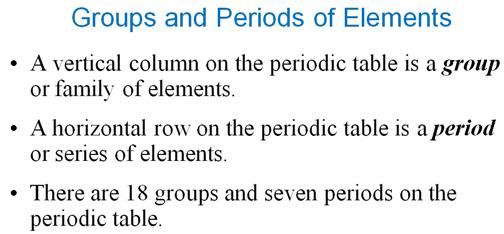


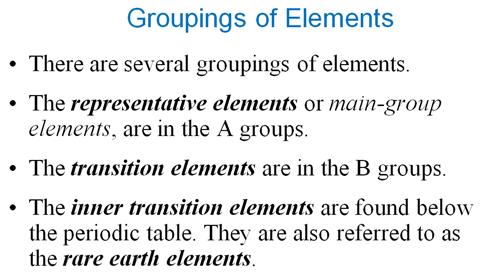
Module
Three Part P: Periodic Chart Identification†††††††††††††† 2 points
Selected symbols
have been placed into the following blank periodic table of elements:
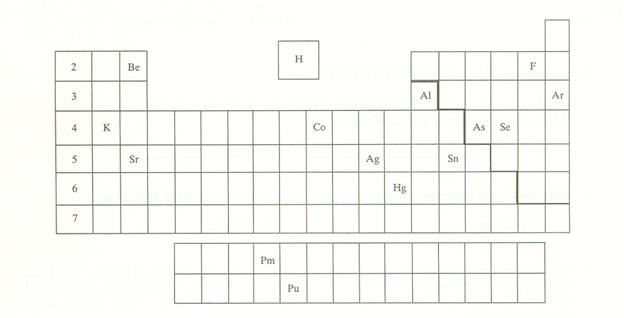
Which symbol in the
above periodic table fits the following description?
_____1. an alkali metal
_____2. A halogen
_____3. an alkaline earth
element
_____4. a noble gas
_____5. A representative element in the fifth
period
_____6. a semimetal
_____7. An element in the lanthanide series
_____8.† an element with the atomic number 13
_____9. an element
filling† a 5d sublevel
_____10. an element with
six valence electrons
_____11. an element
corresponding to: 1s2 2s2 2p6 3s2
3p6 4s2 3d7
_____12. an element with
four valence electrons
_____13. an element in the
actinide series
_____14. the main isotope
of this element has zero neutrons in the nucleus
_____15. a representative
element in the first period of the periodic table
Review
Corwinís 7th †Sections 5.1-5.3 pages 134-138
Additional Questions: p155-6 #8-36
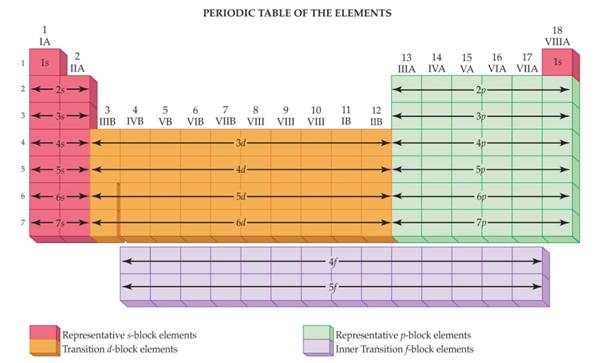
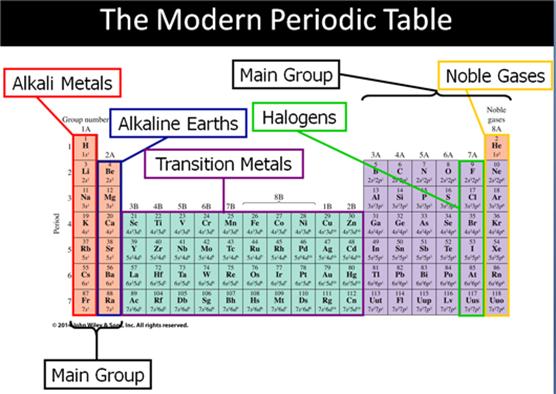
Main Group Elements are also called Representative
Elements




††††††††††