CHM 1025C Module 4 Homework Packet Name:___________________
Module4i: Answers Language of Chemistry/Chemical Bonds (Chapter 11)
A. _____(02) Bond Recognition/Compound Classification-Sections 11.3, 11.5 Answers ac
B. _____(10) Dot Structures of Molecules-Section 11.7/11.8 Answers
_______(12)
Module 4i Total (Seventh Exam)
Module 4ii: Answers Language of
Chemistry/Chemical Bonds (Chapter 6)
C. _____(02) Binary Molecular(Covalent) Compounds-Section 6.4 Answers ac
D _____(02) Binary Ionic Compounds-Section 6.4 Answers
E. _____(05) Polyatomic Ions-Section – section 6.5 Answers e
F. _____(05) Ternary Ionic Compounds-Section 6.5 Answers f
G. _____(02) Binary Acids/ Ternary Oxyacids-Section 6.6 Answers g
H. _____(04) Inorganic Compounds 6.4-6.6 Answers h
_______(20)
Module 4ii Total (Eighth Exam)
_______(14) Module 4 Homework Packet Total Points
Foundations of
College Chemistry, 14th Edition
6 Nomenclature
of Inorganic Compounds 98
6.1
Common and Systematic Names 99
6.2
Elements and Ions 100
6.3
Writing Formulas from Names of Ionic Compounds 103
6.4
Naming Binary Compounds 105
6.5
Naming Compounds Containing Polyatomic Ions 109
6.6
Acids 111
Review
114
Review
Questions 115
Paired
Exercises 116
Additional
Exercises 117
Challenge
Exercise, Answers to Practice Exercises 118
Chapters
5–6 review 119
11 Chemical Bonds: The
Formation of Compounds from Atoms 212
11.1
Periodic Trends in Atomic Properties 213
11.2
Lewis Structures of Atoms 216
11.3
The Ionic Bond: Transfer of Electrons from One Atom to Another 217
11.4
Predicting Formulas of Ionic Compounds 222
11.5
The Covalent Bond: Sharing Electrons 224
11.6
Electronegativity 226
11.7
Lewis Structures of Compounds 229
11.8
Complex Lewis Structures 232
11.9
Compounds Containing Polyatomic Ions 234
11.10
Molecular Shape 235
Review
239
Review
Questions 240
Paired
Exercises 241
Additional
Exercises 243
Challenge
Exercises 244
Answers
to Practice Exercises 245
Chapters
10–11 review 246
Module 4: Part A: Bond Recognition
Read the short
discussion in Corwin’s (7th) sections 12.1-12.4 on pages 341-354 on
the difference between Ionic and covalent bonding. In Hein (14th)
read sections 11.1-11.5, 11.7
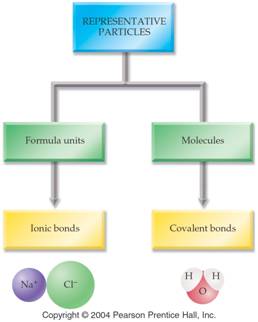
There are three types of chemical bonds:
Ionic,
Covalent, and Metallic.
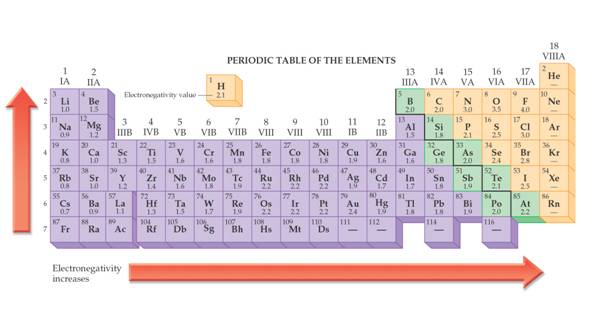
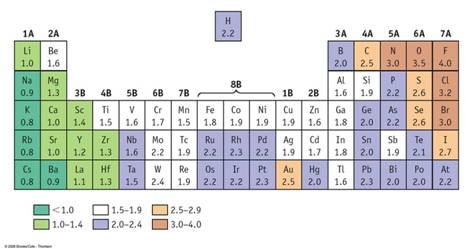
There is a simpler way to predict if two atoms will transfer their electrons or share their electron in pairs making a compound. In Corwin’s 7th edition skip back to Sections 12.6 and 12.7. Read about the Pauling’s Scale of Electronegativity. Corwin Figure 12.9 shows the electronegativity of each element on the periodic chart. This table will be needed in Module Four Part II Bond Polarity. In Hein’s 14th edition, read section 11.6 about electronegativity.
If
the difference in electronegativity
between two atoms is
greater than 1.8 (Corwin), the electrons will transfer from one
atom to the other to make ions and Ionic
Compounds. Ionic (sometimes called Electrovalent) Compounds are also called
salts
and in nature they are called minerals
and in Sports medicine Body Electrolytes. We will over simplify
this concept to say if a metal meets a nonmetal ionic bonds are formed (Just a
Rule of Thumb)(if a table of electronegativity is not included). Hein (14th)
states on page 227 if the difference in electronegativity is greater than 2.0
the bonding is strongly ionic, while less than 1.5 strongly covalent. Then he
states between 1.7-1.9 the bonding will be more ionic than covalent.
For this course, if the difference between the electronegativity of two atoms is less than 1.7 then the two atoms will share electrons in pairs. Two types of sharing bonds are formed. Metallic and Covalent.
Metallic Bonds are
formed when two metals share electrons such as alloys of metals. 24 karat gold
is pure gold and is very soft. But Jewelry is usually 10-18
Karat Gold, meaning that another metal is mixed with gold to make the solid
harder. We will not study Metallic Bonds in this course, but you should
know that two metals share electrons in pairs to make Metallic Bonds.
“Metallic bonding occurs as a result of electromagnetism and describes the electrostatic attractive force that occurs between conduction electrons (in the form of an electron cloud of delocalized electrons) and positively charged metal ions. It may be described as the sharing of free electrons among a lattice of positively charged ions (cations
). In a more quantum-mechanical view, the conduction electrons divide their density equally over all atoms that function as neutral (non-charged) entities.[citation needed] Metallic bonding accounts for many physical properties of metals, such as strength, ductility, thermal and electrical resistivity and conductivity, opacity, and luster.[1][2][3][4]Metallic
bonding is not the only type of chemical
bonding a metal can exhibit, even as a pure substance. For example,
elemental gallium
consists of covalently-bound pairs of atoms in both liquid and solid
state—these pairs form a crystal lattice with metallic bonding between them.
Another example of a metal–metal covalent bond is mercurous ion (Hg2+2).“
Covalent Bonds are formed when two nonmetals bond together. The elements carbon, oxygen, hydrogen, sulfur, nitrogen, phosphorus, chlorine, and bromine will be the main nonmetals studied in drawing dot structures of molecules. Bonds between these nonmetals are always Covalent.
Part A of Module Four should now be easy. Predict what type of
bond will be made if two atoms combine:
In General:
Metal-Metal = Metallic Bond (example: Ag(5)-Au(14)-Cu(5) = 14 Karat Gold)
Metal-Nonmetal = Ionic Bond (example: Na-Cl)
Nonmetal-nonmetal
= Covalent Bond
(example: H2O)
M-4 Required
Homework/Tests/Exercises:
______(32) Online Names/Formulas Homework (Submit separate Goldenrod form on Exam#2 Day)
________(02) Polyatomic Ions Flash Card or Progressive
Polyatomic ion online Homework
________(02) Corwin/Hein
Textbook Chapter 6 Table Polyion Test
______(05) Polyatomic Ions Progressive Test#1 (Best Score of two attempts) Required List
______(05) Polyatomic Ions Progressive Test#2 (Best Score of two
attempts) Required List
______(20) Hard Copy Dot Structure Homework/Lab:
Module
Four: Part A Sample
Bond Recognition 2 points
Using
a periodic chart (Rule of Thumb), predict the bond that would form between the
two elements:
1. Fe-Al ________________
2. P-S ________________
3. C-O ________________
4. B-Cl ________________
5. K-I ________________
For the following element pairs use the electronegativity table below to determine if the bond is ionic or covalent.
6. Na-P ________________
7. Ca-Br ________________
8. Ge-O ________________
9. P-H ________________
10. Be-Cl ________________
Text Reference Sections 12.1-12.2-12.3 + Study Guide: Hein: 11.6 page 227
http://www.fccj.us/chm1025/AssignmentOutline/M4PartA.htm
Module
Four: Part B Dot Structures of Molecules 0 points
Using
a periodic chart draw the electron dot structures of the following molecules:
(Choose One for each
question or the one circled on the paper)
1. NH3 CH4 H2O2 H2O 2.
H2SO4 H3PO4 HClO4 HClO3
Submit these dot
structures as a separate homework
3. HNO3 H2CO3 HNO2 4.
CO2 HCN SO3 SO2
Submit these dot
structures as a separate homework
5. HC2H3O2 H2C2O4 HCHO2
6.
C2H4 C2H2 C3H8 C2H6
carbon to carbon by single
covalent bond bond
carbons to carbon
Submit these dot
structures as a separate homework
7. CH3CH2OH CH3COCH3 CH2O (HCHO)
(carbon to
carbons by single covalent bonds-oxygen attach to carbon)
Submit these dot
structures as a separate homework
8. CH3OCH3 CHONH2 CH3CH2CH2OH CH3CHOHCH3
oxygen
separates the carbons O & N
both bond to C (all three
carbons single bonded and –OH attached to carbon)
Submit these dot
structures as a separate homework
9. CH2NH2COOH CH3CHNH2COOH
carbon to carbons by
single covalent bonds (-NH2 amino on#2 carbon in both above)
Submit these dot
structures as a separate homework
10. CH3COOCH2CH3 HCOOCH3
(-CH2CH3 also
hooks to oxygen in#10, as well as - CH3 )
Submit these dot structures as a separate
lab homework 10 points
See
handout and Corwin Chapter 12 sections 12.4-12.5 for directions; Hein Chapter
11 Sections 11.7, 11.8, 11.9
Drag and Drop Interactive Web Site (Nothing required to turn in):
http://www.lsua.us/chem1001/dragdrop/menu.html
CHM 1025C
Module 4 Homework Packet
Binary Molecular compounds are
explained after the ionic compounds in Corwin (7th) Chapter 6
section 6.7, and inorganic acids are not covered until last in the chapter,
sections 6.8 and 6.9 (Corwin 7th. Hein includes Binary Molecular at
the end of section 6.4 (Page 108 14th) covering all Binary Compounds
first ionic, then molecular.
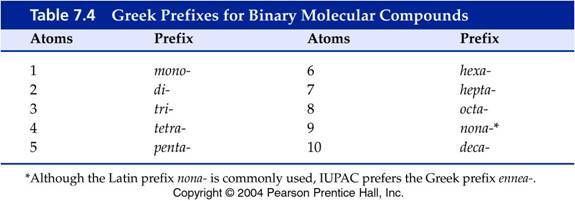
The required Online Binary Covalent Molecular Homework
The
web site is:
C: Binary Molecular Names:
http://www.northcampus.net/Nomenclature/Molecules/25BinaryCovalent.html
C1:
Binary Molecular Formulas:
http://www.northcampus.net/Nomenclature/MoleculeFormula/25BinaryMolecularFormula.html
Here is a brief tutorial for Part
C:
PART C: BINARY COVALENT COMPOUNDS
Both elements are nonmetals attached by covalent bonds. These bonds may be single, double, or triple covalent. Due to the covalent bonding there are many ratios of the same two elements making many different compounds. For this reason, the chemist states how many atoms of each element is present in the chemical formula in the formal name of the compound.
Prefixes are attached to each element to indicate how many. Each student should learn the following prefixes:
MONO
=
ONE
HEXA
= SIX
DI
=
TWO
HEPTA
= SEVEN
TRI
=
THREE OCTA
= EIGHT
TETRA
=
FOUR
NONA
= NINE
PENTA
=
FIVE
DECA
= TEN
The element that is shown first in the chemical formula is written first using the proper prefix to indicate how may atoms of that element is contained in the compound. If there is only one atom of that element it is often found without the prefix mono. If you leave the prefix off then it is understood that you mean mono.
The element which is written second in the chemical formula is written second in the chemical name, but in addition to the prefix indicating how many, the suffix of the element’s name is changed to -ide.
carbon becomes carbide chlorine becomes chloride
sulfur becomes sulfide oxygen becomes oxide
hydrogen becomes hydride nitrogen becomes nitride
Therefore, the following formulas of binary compounds would be spoken:
CCl4 carbon tetrachloride
SO2 sulfur dioxide
CO2 carbon dioxide
N2O3 dinitrogen trioxide
BH3 boron
trihydride
We use common names for NH3, and H2O. What would be their correct binary molecular names? Methane, CH4, is the organic name for CH4, what would its inorganic name be?
For more practice on Corwin page 185 try problems 45 thru 48 for binary nonmetal compounds.
Module Four:
Part C Binary Molecular Compounds 2 points
Using
a periodic chart write the names or formulas of the following compounds
depending on whether the formula or name is given:
Homework
Packet Sample test: answer on grading outline
1. CO
____________________
2. SO3 _____________________
3. N2O5 _____________________
4. N2O7 _____________________
5. N2O _____________________
6. Phosphorus pentachloride _________
7. Boron trifluoride _________
8. Carbon dioxide _________
9. Sulfur Trioxide _________
10. Carbon Tetrachloride _________
Textbook Reference: Corwin Chapter 6 Section 6.7 Optional End of Chapter p185
#45-48
Hein
Section 6.4 page
108
Online
Homework (2 Points Each Required):
C:
Binary Molecular (Covalent) Homework: http://www.northcampus.net/Nomenclature/Molecules/25BinaryCovalent.html
C1.
Binary Molecular (Covalent) Formulas: http://www.northcampus.net/Nomenclature/MoleculeFormula/25BinaryMolecularFormula.html
Submit
grades on separate grading Sheet(goldenrod) when
taking Module 4 Exam
Online
Study Guide:
http://www.fccj.us/chm1025/AssignmentOutline/M4PartC.htm
CHM
1025C Module 4 Homework Packet
Module 4: PART D:
BINARY (IONIC) COMPOUNDS
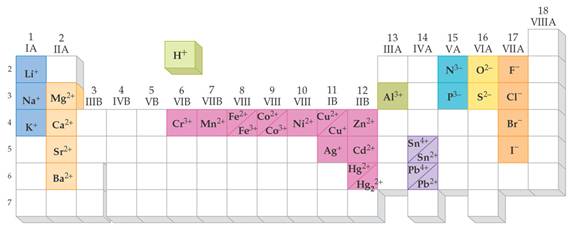
Most Common Ionic
Charges for Monatomic Ions
PART D:
BINARY (IONIC) COMPOUNDS
The element written first
in either the name or the formula is a metal. The element written second
is a nonmetal. Salts are metallic and nonmetallic ionic
compounds. There are no molecules of salts-just macro ionic
lattices. Name the metallic element.
If the metallic element has more than one ionic state, write a ROMAN NUMERAL after the element’s name (In Parathesis) to indicate which charge state the metallic element is using to form the compound.
Drop the suffix off the nonmetal’s name and add -ide which indicates the salt is binary
(exceptions: cyanide & hydroxide which are polyatomic ions).
No prefixes are used to indicate how many atoms are present in the formula.
Examples:
NaCl Sodium Chloride (table salt)
Al2O3 Aluminum oxide
: FeS Iron(II) sulfide (Note: No space between the metal and the parenthesis)
Fe2O3 Iron(III) oxide (rust)
To write the formula from the name of the salt use the following procedure:
(a) Write the symbols
(or formulas for radicals) of the ions represented
For Example:
Calcium nitride
(a)
Ca N
(b) Use the periodic chart to write the ion charge of each element (or polyatomic ion) as superscripts:
Ca+2 N-3
(c ) Find the L.C.M. (Least common multiple) of the positive and negative charge.
The LCM is the smallest number that both charges will decide into evenly. The LCM is the total electrons transferred. Therefore, it represents the total positive charge created by the metallic ions and the total negative charge created by the nonmetallic ions. This may be proved by drawing the dot structure of the compound showing all electrons transferred.
The LCM of +2
and -3 is 6, therefore 6 e-1
are transferred creating a total positive charge of +6, and the total negative charge
of -6
--> 6e-1-->
Ca+2
N-3
(d (d) Divide the LCM by the positive
charge, this dividend will represent the subscript behind the metallic ion
in the formula.
+6 divided by +2 = 3;
therefore half of the formula is: Ca3Nx
(e) Divide the LCM by the negative charge, this dividend will represent the number of nonmetallic ions in the formula.
-6 divided by -3 = 2; therefore the other half of the formula is: Ca3N2
Example:
Potassium phosphide
Write Symbols and the Charges:
K+1 P -3
LCM:
3
Balance the chemical
formula:
K3P
In addition to working the sample tests, you may want to practice on writing the names and formulas for Ionic Compounds.
On Corwin (7th) pages 184-5, questions 19 thru 34 are also good practice. Hein (14th) page 116
You must complete the online homework for 3 points each:
D. Binary Ionic Names:
http://www.fscj.me/Nomenclature/BinarySalts/25BinaryIonicJT.html
D1. Binary Ionic Formulas:
http://www.northcampus.net/Nomenclature/BinaryIonicFormula/25BinaryIonicFormula.html
Module
Four: Part D Binary Ionic
Compounds 2 points
Using
a periodic chart, write the names or the balanced formulas for the following
compounds depending on whether the formula or the name is given:
1. Copper II phosphide _________ (Cupric phosphide)
2. Iron III Oxide (rust) _________ (Ferric Oxide)
3. Lead IV sulfide _________ (Plumbic sulfide)
4. Sodium chloride _________
5. Tin II fluoride (in toothpaste) _________ (Stannous Fluoride)
6. MgCl2 ________________________
7. NiF2 ________________________
8. K3N ________________________
9. Al2O3 ________________________
10.
CuBr
________________________
Optional: Also Work Questions #25-34 P 184-185 in Corwin 7th
textbook Reference Section 6.4-6.5 Corwin text
Online
Homework (3 Points
Each):
D:
Binary Ionic Compound Homework: http://www.fscj.me/Nomenclature/BinarySalts/25BinaryIonicJT.html
D1.
Binary Ionic Formulas:
http://www.northcampus.net/Nomenclature/BinaryIonicFormula/25BinaryIonicFormula.html
Submit grades on separate
grading Sheet when taking Module 4 Exam
Online Study Guide:
http://www.fccj.us/chm1025/AssignmentOutline/M4PartD.htm
CHM
1025C Module 4 Homework Packet
Corwin’s (7th) Ion
Flowchart-Chapter 6
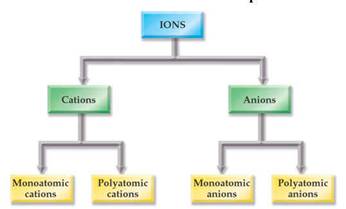
From Chapter 6 Corwin (7th) (Chapter 5 Hill), Chapter 6 Hein (14th)Monoatomic Anions or Cations can be predicted the position the element resides on the periodic chart, if the ion come from a Representative Element (IA-VIIIA) or by its name if it is a transitional metal with several different charges. Below is Corwin (7th) Figure 6.3 demonstrating common cations and anions:
Periodic
Table of Selected Ions
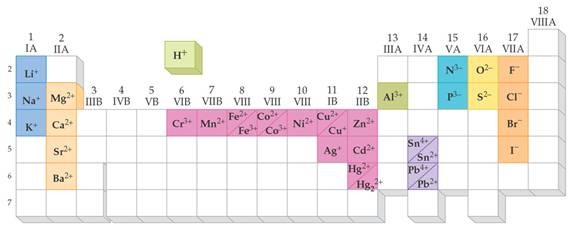
Note the charges for groups IA,
IIA,
You should practice: M-3 Part F, then try M-4 Part D and write the formulas for Binary Ionic Compounds.
Sections 6.4 and 6.5 of the Corwin (7th) text describes the
process. For the Hein Textbook (14th)
look at sections 6.1, 6.2, 6.3 and 6.4. Then you should try the web site for
homework points Naming Binary Ionic
Compounds (if you already have not done so!):
http://www.fscj.me/Nomenclature/BinarySalts/25BinaryIonicJT.html
and writing the formulas of Binary Salts
(if you have not already done so):
http://www.northcampus.net/Nomenclature/BinaryIonicFormula/25BinaryIonicFormula.html
You should practice Questions
#19-#34 at the end of Chapter 6 (Corwin 7th) for more practice.
Almost all chemistry
textbooks have sections dedicated to polyatomic ions and include a list of
common ions.
What is
a polyatomic ion?
A group of atoms bound together (covalent bonds)
that bears an overall negative or positive charge.
Corwin (7th) suggests that you use flash cards listing the name on one side and the formula with its charge on the other to aide your memorization of these formulas. Most chemistry teachers require you to know some of the common polyatomic ions by the end of the course whether it is from repetition of use with a help table or from memory from the first day of introduction. Below are tables from various chemistry books used:
Polyatomic Ion
Charts from Textbooks
McMurray: Table
3.2 Corwin: Table 7.03
Silverberg: Table
2.5 Tillery: Table
9.3
Kotz: Table 3.1 Hill: Table
5.04
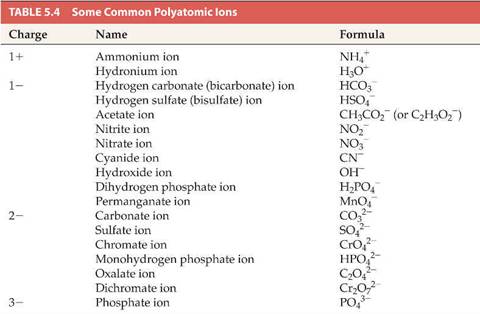
Here is a sample polyatomic ion table:
Hill’s
Table 5.4 (and Hill suggest for you to memorize
the entire table):
After you start memorizing, during the
course the formulas may be swimming in you head and
the charges too. To write balance Ternary Ionic Compounds, you must be able to
write the formula and the charge of each polyatomic ion required.
Corwin suggests there is only
one (Hill has two) common polyatomic Cation(s) and
both end in –ium suffix. He notes most of the Anions have an –ate suffix, while a few
have –ite,
and two have –ide in their name. How do we accomplish this list?
Knowing dot structures of
polyatomic ions (Corwin Chapter 12 section 12.5), and some keen observations
you can boil it down to six questions:
1. What is the formula
for the –ate polyatomic ion?
2. What is the charge
on –ate polyatomic ion?
3. What happens when you attach hydrogen atom(s) to the polyatomic
2- and 3-
anions?
4. What does –ite mean?
5. How do the hypo-
and per- prefixes
apply to polyatomic ions?
6. What are the two –ide
polyatomic ions and two -ium positive Anions?
Your First task is to
memorize the formulas and the charges for the polyatomic ions in your text book
for a short test:
Progressive Polyatomic
Ions Corwin (0 points))
Write the
formula and the charge for the following polyatomic ions: Corwin(Table
6.3) 19 points
|
Name |
Formula with charge |
|
Acetate |
|
|
Ammonium |
|
|
Carbonate |
|
|
Chlorate |
|
|
Chlorite |
|
|
Chromate |
|
|
Cyanide |
|
|
Dichromate |
|
|
Hydrogen Carbonate |
|
|
Hydrogen sulfate |
|
|
Hydroxide |
|
|
Hypochlorite
|
|
|
Nitrate |
|
|
Nitrite |
|
|
Perchlorate |
|
|
Permanganate |
|
|
Phosphate |
|
|
Sulfate |
|
|
Sulfite |
|
Progressive
Polyatomic Ions Hein (2 points)
Write the formula and the charge for the following
polyatomic ions: Hein(Table 6.5 page 109) 18 points
|
Name |
Formula
with charge |
|
Acetate |
|
|
Ammonium |
|
|
Arsenate |
|
|
Carbonate |
|
|
Chlorate |
|
|
Chromate |
|
|
Cyanide |
|
|
Dichromate |
|
|
Hydrogen Carbonate |
|
|
Hydrogen sulfate |
|
|
Hydroxide |
|
|
Nitrate |
|
|
Nitrite |
|
|
Permanganate |
|
|
Phosphate |
|
|
Sulfate |
|
|
Sulfite |
|
So: it is time for you to discover, what I saw over 50 years ago. It is not in any textbook. The books just say know or memorize these tables. Go to:
http://www.fccj.us/PolyatomicIons/polyionformula.html
When you go to the siute
above (which looks like the image below), click on the X for each polyatomic
ion and note if the # of oxygens is three or four in
the formula.
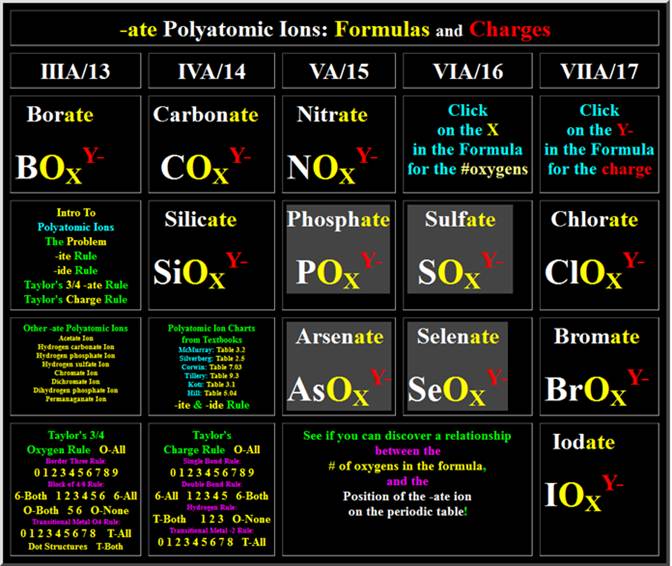
To
expose the threes and the fours in the lower left hand corner (Taylor’s ¾ rule)
click the numbers 0,1…8,9 Border three rule, then
1,2..5,6 in the box of six rule. Also do the 0,1…7,8
Transitional O4 Rule.
Taylor’s
¾ rule is summarized at:
http://www.fccj.us/PolyatomicIons/Taylor34OxygenRuleHandout.htm
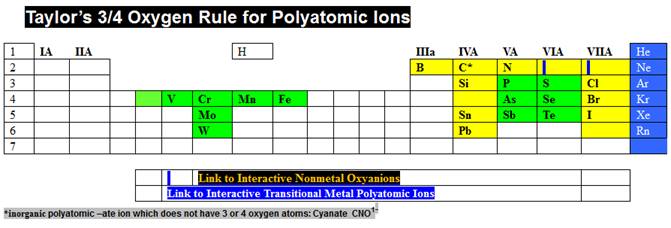
Then
do the same for the box just to the right of Taylor’s ¾ Rule, and discover
Taylor’s Charge Rule.
Taylor’s
Charge Rule is summarized at:
http://www.fccj.us/PolyatomicIons/TaylorChargeRuleHandout.htm
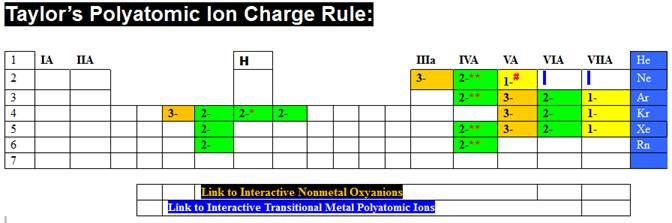
The
story behind how your instructor related the periodic table to a long list of polyions, read the abstract for his talk at 2YC3:
http://www.fccj.us/PolyatomicIons/2YC3HowTeachPolyatomiIonsInChemistry.htm
Now
comes the big task!
You
may either memorize 55 polyatomic ions or learn to read the periodic table with
six rules and be able to write formulas and the charges for the required 1025
list:
http://www.fscj.me/PolyatomicIons/25MemorizeList.htm
Either
make a hard copy set of polyatomic ion flash cards or practice the 65
polyatomic ions Flash Card web site for 2 points at:
http://www.fscj.me/Nomenclature/PolyatomicIonFormula/ProgressivePolyatomicIonFormula.html
Module
Four: Part E Polyatomic Ions 2 points
Using
a periodic chart write the names or formulas of the following polyatomic ions
depending on whether the formula or name is given:
1. CO32- _____________________
2. SO32- _____________________
3. PO33- _____________________
4. ClO31- _____________________
5. NO31- _____________________
6. Hydroxide ________
7. Ammonium ________
8. Hypochlorite ________
9. Nitrite ________
10. Phosphate ________
Textbook (Corwin 7th) Reference: Chapter
6 Section 6.3 Table 6.3 Optional End of Chapter p184 #13-18
Hein (14th): Section 6.5 Memorize
the formulas and charges of Hein Table 6.5 (18 ions)
Online
Required Homework
(3 Points Each):
E:
Polyatomic Ion Names Homework: http://www.northcampus.net/Nomenclature/PolyatomicIon/25PolyatomicIon.html
E1.
Polyatomic Ion Formulas: http://www.northcampus.net/Nomenclature/PolyatomicIonFormula/25PolyatomicIonFormula.html
Submit
grades on separate grading Sheet (Goldenrod) when taking Module 4 exam or
download the form from:
http://www.fscj.me/chm1025/NomenclatureGradingLabForm.htm
CHM
1025C Module 4 Homework Packet
In chemistry,
a ternary compound is a compound containing three different elements. An
example of this is sodium phosphate, Na3PO4.
The sodium ion has a charge of 1+ and the phosphate ion has a charge of 3-.
Therefore, three sodium ions are needed to balance the charge of one phosphate
ion. Another example of a ternary compound is calcium carbonate
. In naming and writing the formulae for ternary compounds, we follow
rules that are similar to binary compounds.(CaCO3).
Ste that uses least common multiple balance method:
http://web.tenafly.k12.nj.us/chemquest2/ternary_compounds.htm
Sites (You-tubes) that use the crossing method(UGH):
You-Tube: http://www.youtube.com/watch?v=8eJtYffLWKc
Another You-Tube: http://www.youtube.com/watch?v=cXyxrzUw99A
Module Four: Part F Ternary Ionic Compounds 2 points
Using
a periodic chart write the names or formulas of the following compounds
depending on whether the formula or name is given:
1. Na2CO3 _____________________
2. K2SO4 _____________________
3. (NH4)3PO4 _____________________
4. Ca(ClO3)2 _____________________
5. CuNO3 _____________________
6. Aluminum Hydroxide ____________
7. Ammonium carbonate ____________
8. Sodium Hypochlorite ____________
9. Magnesium Nitrate ____________
10. Iron III sulfite _____________
Corwin Text Sections 6.4 and 6.6
Optional
Additional Homework: p 185 Q #35-44
Online
Homework (3 Points Each Required):
F:
Ternary Ionic Compound Names Homework: http://www.northcampus.net/Nomenclature/TernarySalts/25ternaryIonic.html
F1.
Ternary Ionic Compound Formulas: http://www.northcampus.net/Nomenclature/TernarySaltFormula/25ternaryionicformula.html
Submit
grades on separate grading Sheet when taking M-4 Exam
CHM
1025C Module 4 Homework Packet
Module 4 Part G: Binary/Ternary Acids
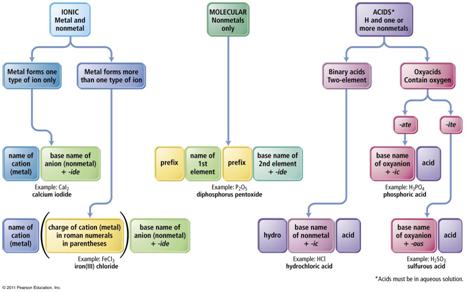
What is an acid?
A substance that releases hydrogen ions (H+) when dissolved in water. Inorganic formulas of acids have ionizable hydrogen(s) written first in the formula.
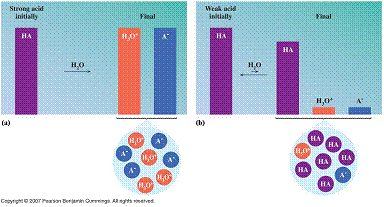
Strong Acids Weak Acids
Strong acids ionize 100% in a water solution, while Weak Acids
ionize
less than 5% in a water solution.
There are Binary/Ternary Acid online homeworks for your practice for M-4 Part G:
G: Binary/Ternary Acid Names:
http://www.northcampus.net/Nomenclature/Acids/25Acids.html
G1: Binary/Ternary Acid Names:
http://www.northcampus.net/Nomenclature/AcidFormulas/25AcidFormulas.html
( Chapter 6 Bishop
Sections 6.3-6.4 )give you instructions for naming and writing formulas of
acids. );
(Chapter 6 Corwin 7th covers
binary acids in section 6.8; while section 6.9 covers ternary acids.) (Hein 14th
covers acids in section 6.6
A brief tutorial for names and formulas of acids follows:
If hydrogen is written first in a chemical formula, there is two
ways to name the compound. As a pure
molecular compound or as an aqueous acid:
If the compound is a pure molecular compound then you name it just as if it were an ionic compound:
HCl
hydrogen chloride
HClO
hydrogen hypochlorite
HClO2
hydrogen chlorite
HClO3
hydrogen chlorate
HClO4
hydrogen perchlorate
H3PO4
hydrogen phosphate
H2CO3
hydrogen carbonate
H2SO4
hydrogen sulfate
H2SO3
hydrogen sulfite
HC2H3O2
hydrogen acetate
H2C2O4 hydrogen
oxalate
HBr hydrogen bromide
HF hydrogen fluoride
Writing hydrogen
first in a chemical formula indicates that when you dissolve the compound
in water, a water molecule has the ability to pull the hydrogen off (from strong electronegative elements like
oxygen) the molecule HXO3 and creating hydronium
ions, H3O1+ and a negative ion XO31-
(cation).
The way you indicate this ionic solution is to write the formula followed by (aq) meaning a water solution: HXO3 (aq) .
The
first step is to drop the first word hydrogen and
add a second word acid:
HCl hydrogen chloride acid (aq)
HClO hydrogen hypochlorite acid (aq)
HClO2
hydrogen chlorite acid (aq)
HClO3
hydrogen chlorate acid (aq)
HClO4
hydrogen perchlorate acid (aq)
H3PO4
hydrogen phosphate acid (aq)
H2CO3
hydrogen carbonate acid (aq)
H2SO4
hydrogen sulfate acid (aq)
H2SO3 hydrogen sulfite acid (aq)
HC2H3O2
hydrogen acetate acid (aq)
H2C2O4 hydrogen oxalate acid (aq)
HBr hydrogen bromide acid (aq)
HF hydrogen fluoride acid (aq)
The next step is to drop the suffix from the cation and make the following substitution with another suffix:
Change the -ate to -ic
Change the -ite to -ous
but the instead of coming up with a third suffix for -ide , they reused the -ic for -ide and added a prefix hydro- (Do not get this confused with the prefix hypo- which means 'under'.)
HCl hydrochloric acid (aq)
HClO hypochlorous acid (aq)
HClO2 chlorous acid (aq)
HClO3
chloric acid (aq)
HClO4
perchloric acid (aq)
H3PO4
phosphoric acid (aq) (Put the -or- syllable
back in the name)
H2CO3
carbonic acid (aq)
H2SO4
sulfuric acid (aq) (Put the -
H2SO3 sulfurous acid (aq)
(Put the -
HC2H3O2
acetic acid (aq)
(Notice
the three hydrogens written after carbon are NOT ionizable and not written first in the formula)
H2C2O4 oxalic acid (aq)
HBr hydrobromic acid (aq)
HF hydrofluoric acid (aq)
On Corwin 7th
page 185 Questions 49-56 will give you more practice on writing names and
formulas of acids.
At the end
of chapter 6 Hein 14th exercises 17, 18, 19, and 20 pages 116-117
are additional acid nomenclature problems.
Module Four: Part G Binary/Ternary Acids 2 points
Using
a periodic chart write the names or formulas of the following compounds
depending on whether the formula or name is given:
1. HCl _____________________
2. H2SO4 ____________________
3. HNO3 _____________________
4. HNO2 ___________________
5. H2CO3 ___________________
6. Hypochlorous
acid _________
7. Phosphoric acid _________
8. Sulfurous acid _________
9. Perchloric
acid _________
10. Hydrofluoric acid ________
Corwin 7th Text Sections 6.8 and 6.9
Optional
Additional Homework: p 185 Q #49-56
Online
Homework (2 Points Each Required):
G:
Binary/Ternary Acid Names Homework: http://www.northcampus.net/Nomenclature/Acids/25Acids.html
G1.
Binary/Ternary Acid Formulas:
http://www.northcampus.net/Nomenclature/AcidFormulas/25AcidFormulas.html
Submit
grades on separate grading Sheet when taking M-4 Exam
Online Study Guide:
http://www.fccj.us/chm1025/AssignmentOutline/M4PartG.htm
CHM
1025C Module 4 Homework Packet
Module Four: Part H Inorganic Compounds 2 points
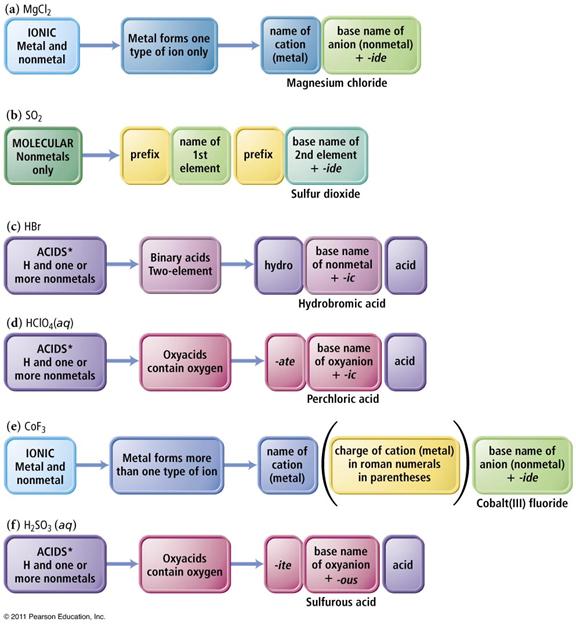
The key to deciding which system to use in Part H is to look at the element written first.
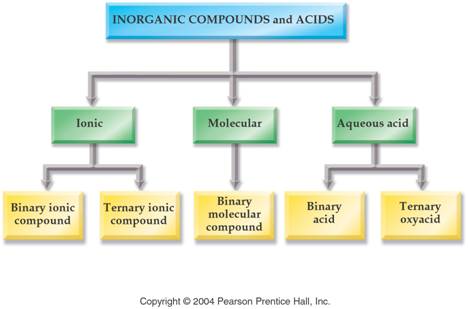
1. If a Metal is written first (or a polyatomic ion), then use the rules for ionic compounds (salts).
2. If a nonmetal is written first, then use the Covalent/Molecule System with prefixes. (If the compound is Organic Nomenclature of Organics is covered in Chapter 11, but for now use the prefix system of binary molecular nomenclature.
3. If hydrogen
is written first (and it is in aqueous solution) then name it as an Acid
CHM
1025C Module 4 Homework Packet
Module Four: Part H Inorganic Compounds 2 points
Using
a periodic chart write the names or formulas of the following polyatomic ions
depending on whether the formula or name is given:
1. H2CO3 _____________________
2. MgSO4 _____________________
3. Ca3(PO3)2 ____________________
4. HClO3 _____________________
5. SO3 _____________________
6. Fe2O3 _____________________
7. Aluminum Hydroxide ____________
8. Ammonium chloride _____________
9. Sodium Hypochlorite _____________
10. Nitrogen dioxide _____________
11. Calcium Phosphate ____________
12. Sulfuric acid ____________
Corwin Text Sections 6.1 through 6.9
Optional
Additional Homework: p 185-6 General Exercises;
Hein 6.3-6.6 End of chapter pages 116-117 Q#
7-16
Online
Homework (3 Points Each Required):
H:
Inorganic Compound Names Homework: http://www.northcampus.net/Nomenclature/Inorganic/25inorganic.html
H1.
Inorganic Compound Formulas:
http://www.northcampus.net/Nomenclature/InorganicFormula/25inorganicFormula.html
Submit
grades on separate grading Sheet when taking M-4 Exam
Online Study Guide:
http://www.fccj.us/chm1025/AssignmentOutline/M4PartH.htm