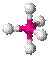Module Four Part III: Chemical Bonding & Molecular Structure Module 4iii: Chemical Bonding & Molecular Structure (Chapters 11)
L. ____ (03) Bond Angles/Bond Lengths-Section 11.10 Answers
N. ____ (03) Geometry of Molecules-Section 11.10 Answers
O. ____ (03) Polarity of Molecules-Section 11.6 Answers
______(09) Module4iii Total (Fourteenth Exam)
Reference:
VSEPR Video:
http://www.lsua.info/chem1001/VSEPR/VSEPRtheory.wmv
What is VSEPR?
VSEPR stands for Valence Shell Electron Pair
Repulsion. It's a complicated acronym, but it means something that's not
difficult to understand. Basically, the idea is that covalent bonds and
lone pair electrons like to stay as far apart from each other as possible under
all conditions. This is because covalent bonds consist of electrons, and
electrons don't like to hang around next to each other much because they have
the same charge.
This VSEPR thing explains why molecules have
their shapes. If carbon has four atoms stuck to it (as in methane), these
four atoms want to get as far away from each other as they can. This
isn't because the atoms necessarily hate each other, it's because the electrons
in the bonds hate each other. That's the idea behind VSEPR.
What
is a Bond Angle?
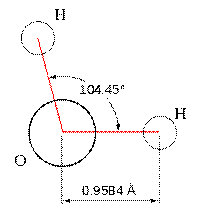
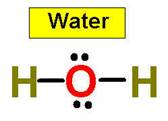
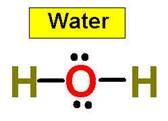
Lets look at the water molecule:
VSEPR table
The bond angles in
the table below are ideal angles from the simple VSEPR
theory, followed by the actual angle for the example given in the
following column where this differs. For many cases, such as trigonal pyramidal and bent, the actual angle
for the example differs from the ideal angle, but all examples differ by
different amounts. For example, the angle in H2S (92°) differs from
the tetrahedral angle by much more than the angle for H2O (104.5°)
does.
|
Bonding electron pairs |
Lone pairs |
Electron domains (Steric
#) |
Shape |
Ideal bond angle (example's bond
angle) |
Example |
Image |
|
2 |
0 |
2 |
180° |
|||
|
3 |
0 |
3 |
120° |
|||
|
2 |
1 |
3 |
120° (119°) |
|||
|
4 |
0 |
4 |
109.5° |
|||
|
3 |
1 |
4 |
107° |
|||
|
2 |
2 |
4 |
bent |
109.5° (104.5°) |
||
|
5 |
0 |
5 |
90°, 120°, 180° |
|||
|
4 |
1 |
5 |
180°, 120°, 90° (173.1°, 101.6°) |
|||
|
3 |
2 |
5 |
90°, 180° (87.5°, < 180°) |
|||
|
2 |
3 |
5 |
linear |
180° |
||
|
6 |
0 |
6 |
90°, 180° |
|||
|
5 |
1 |
6 |
90° (84.8°), 180° |
|||
|
4 |
2 |
6 |
90°, 180° |
|||
|
7 |
0 |
7 |
90°, 72°, 180° |
|||
|
6 |
1 |
7 |
72°, 90°, 144° |
XeOF5− |
||
|
5 |
2 |
7 |
72°, 144° |
|||
|
8 |
0 |
8 |
||||
|
9 |
0 |
9 |
|
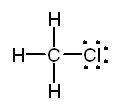
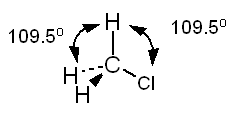
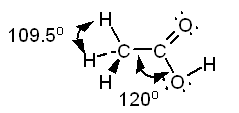
Example Predict all bond angles in the
following molecules. a. CH3Cl b.
CH3CNl c. CH3COOH Solution a. The Lewis structure of
methyl chloride is:
In the Lewis structure of CH3Cl
carbon is surrounded by four regions of high electron density, each of which
forms a single bond. Based on the VSEPR model, we predict a tetrahedral
distribution of electron clouds around carbon, H - C - H and H - C - Cl bond angles of 109.5°, and a tetrahedral shape for the
molecule. Note the use of doted lines to represent a bond projecting behind
the plane of the paper and a solid wedge to represent a bond projecting
forward from the plane of the paper.
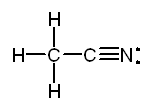
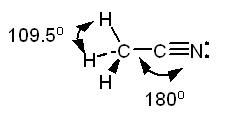
b. The Lewis structure of acetonitrile, CH3CN is:
The methyl group, CH3-,
is tetrahedral. The carbon of the -CN group is in the middle of a straight
line stretching from the carbon of the methyl group through the nitrogen.
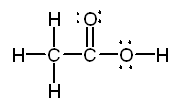
c. The Lewis structure of
acetic acid is:
Both the carbon bonded to three
hydrogens and the oxygen bonded to carbon and
hydrogen are centers of tetrahedral structures. The
central carbon will have 120 7deg bond angles.
The geometry around the first
carbon is tetrahedral, around the second carbon atom is trigonal
planar, and around the oxygen is bent. |
Module
Four: Part L Bond Angles 03
points
What
is the bond Angle in the following structures:
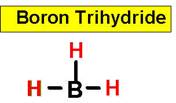
___1. 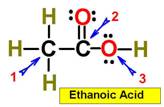 ___4.
___4.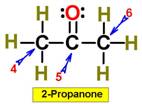
___2. ___5.
___3. ___6.
___7. 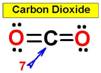 ___8.
___8. 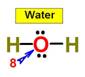 ____9.
____9.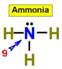
___10. 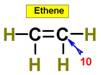 ____11.
____11. 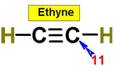
___12. 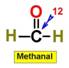 ___13.
___13. 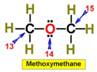 ___16.
___16.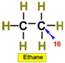
___14.
___15.
___17.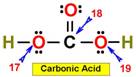 ___20.
___20. 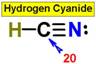
Bonus:
___21. 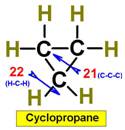 ___23.
___23. 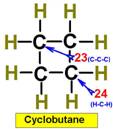
___22. ___24.
Steric Numbers do not predict bond angles within rings of carbons
Types of molecular structure
Some common shapes of simple molecules include:
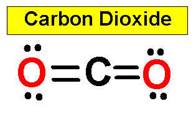
- Linear:
In a linear model, atoms are connected in a straight line. The bond angles
are set at 180°. A bond angle is very simply the geometric angle between
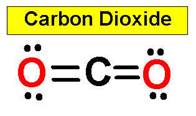
two adjacent bonds. For example, carbon dioxide and nitric
oxide have a linear molecular shape.
- Trigonal
planar: Just from its name, it can
easily be said that molecules with the trigonal
planar shape are somewhat triangular and in one plane (flat).
Consequently, the bond angles are set at 120°. An example of this is boron trifluoride.
- Bent:
Bent or angular molecules have a non-linear shape. A good example is water,
or H2O, which has an angle of about 105°. A water molecule has
two pairs of bonded electrons and two unshared lone pairs.
- Tetrahedral: Tetra-
signifies four, and -hedral relates to a
face of a solid, so "tetrahedral"
literally means "having four faces". This shape is found when
there are four bonds all on one central atom,
with no extra unshared electron
pairs. In accordance with the VSEPR
(valence-shell electron pair repulsion theory), the bond angles between
the electron bonds are arccos(−1/3) = 109.47°.
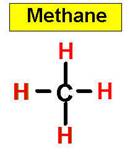
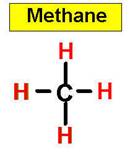
An example of a tetrahedral molecule is methane
(CH4).
- Octahedral: Octa- signifies eight, and -hedral relates to a face of a solid, so "octahedral"
literally means "having eight faces". The bond angle is
90 degrees. An example of an octahedral molecule is sulfur hexafluoride
(SF6).
- Pyramidal: Pyramidal-shaped molecules have pyramid-like shapes. Unlike the linear and trigonal
planar shapes but similar to the tetrahedral orientation,
pyramidal shapes require three dimensions in order to fully separate the
electrons. Here, there are only three pairs of bonded electrons, leaving
one unshared lone pair. Lone pair bond pair repulsions change the angle
from the tetrahedral angle to a slightly lower[citation needed]
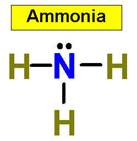
value. An example is NH3 (ammonia).
Common shapes you should
know
There are a whole
bunch of common shapes you need to know to accurately think of covalent
molecules. Here they are:
- Tetrahedral: Tetrahedral
molecules look like pyramids with four faces. Each point on the
pyramid corresponds to an atom that's attached to the central atom.
Bond angles are 109.5 degrees.
- Trigonal
pyramidal: It's like a tetrahedral molecule, except
flatter. It looks kind of like a squished pyramid because one of the
atoms in the pyramid is replaced with a lone pair. Bond angles are
107.5 degrees (it's less than tetrahedral molecules because the lone pair
shoves the other atoms closer to each other).
- Trigonal
planar: It looks like the hood ornament of a Mercedes
automobile, or like a peace sign with that bottom-most line gone.
The bond angles are 120 degrees.
- Bent: They look, well, bent.
Bond angles can be either 118 degrees for molcules
with one lone pair or 104.5 degrees for molecules with two lone pairs.
- Linear: The atoms in the molecule are in
a straight line. This can be either because there are only two atoms
in the molecule (in which case there is no bond angle, as there need to be
three atoms to get a bond angle) or because the three atoms are lined up
in a straight line (corresponding to a 180 degree bond angle).
- There are other types, but we won't worry
about them.
Module Four- Part N: Geometry of Molecules 03 points
Use the dot/stick structures on the Part L page to state the geometry of the molecules:
Bent Linear Trigonal Planer Planer Trigonal Pyramidal Tetrahedral
Trigonal-bipyramidal Square Planer Seesaw T-shaped Octahedral
|
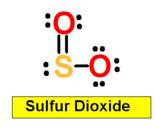
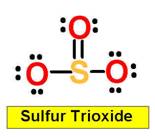
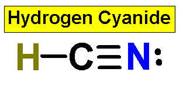
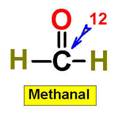
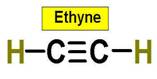
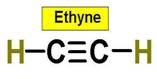
_____________1. H2O _____________2. CO2 _____________3. C2H4 _____________4. SO2 _____________5. SO3 _____________6. HCN _____________7. CH4 _____________8. NH3 _____________9. CH2O _____________10.
C2H2 _____________Bonus. PF5 _____________Bonus SF6 |
|
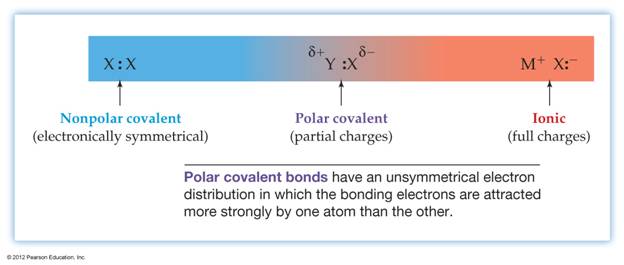
Polar
Covalent Bonds
Covalent bonds result from the sharing of valence electrons.
Often, the two atoms do not share the electrons equally. That is, one of
the atoms holds onto the electrons more tightly than the other.
When one of the atoms holds the shared electrons more tightly, the bond
is polarized.
A
polar covalent bond is one in which the electrons are not shared equally
Electronegativity
Each
element has an innate ability to attract valence electrons.
Electronegativity is the ability of an atom to
attract electrons in a chemical bond.
Linus
Pauling devised a method for measuring the electronegativity of each of the
elements.
Fluorine
is the most electronegative element.
Electronegativity
increases as you go left to right across a period.
Electronegativity
increases as you go from bottom to top in a family.
Electronegativity: The ability of an atom in a
molecule to attract the shared electrons in a covalent bond.
Electronegativity Differences
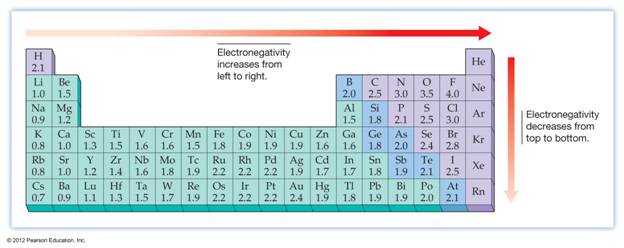
The
electronegativity of H is 2.1; Cl is 3.0.
Since
there is a difference in electronegativity between the two elements (3.0 2.1
= 0.9), the bond in H Cl is polar.
Since
Cl is more electronegative, the bonding electrons are
attracted toward the Cl atom and away from the H
atom. This will give the Cl atom a slightly negative
charge and the H atom a slightly positive charge.
Nonpolar Covalent Bonds
What
if the two atoms in a covalent bond have the same or similar electronegativities?
The
bond is not polarized and it is a nonpolar
covalent bond. If the electronegativity difference is less than 0.5, it
is usually considered a nonpolar bond.
The
diatomic halogen molecules have nonpolar covalent
bonds.
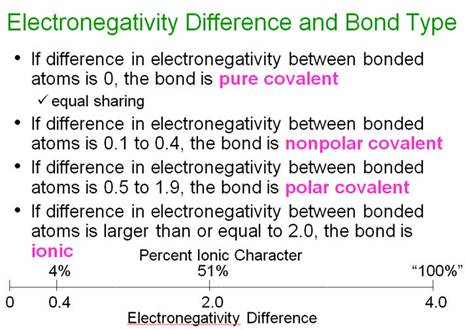
Module Four II- Part O: Polarity of Molecules 03 points
Use the dot/stick structures and sketch the molecule in three dimensions. Then draw the dipoles for each bond to state if the molecule is polar or nonpolar:
Electronegativities: F=4.0; O=3.5; N=3.0; Cl=3.0; Br=2.7; C=2.5; S=2.5; P=2.1; H=2.1
|
_____________1.
H2O _____________2.
CO2 _____________3.
C2H4 _____________4. C2H2 _____________5. SO2 _____________6. SO3 _____________7.
CH4 _____________8.
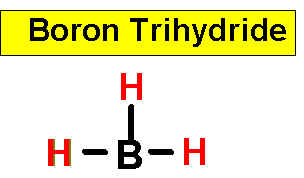
NH3 _____________9.
BH3 _____________10. HCN
_____________Bonus. PCl5 _____________Bonus SCl6 |
|
Diatomic
Halogen Molecules

Chart
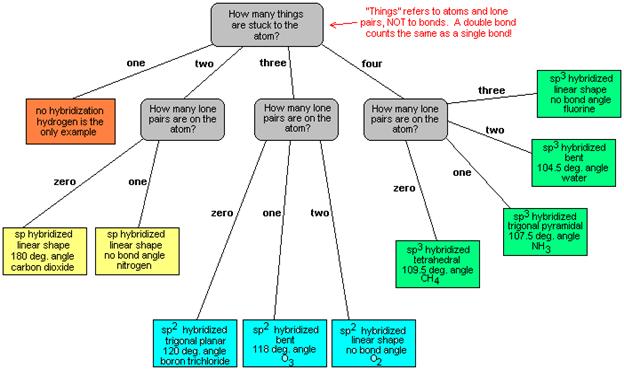
of Hybridization Bonding: