CHM 1032C Tentative Grading
Outline Fall
2015
Chapter
4: Molecular Compounds Homework Packet
B. _____(04*) Dot Structures of Molecules-Section 4.7 Answers
C. _____(02) Binary Molecular(Covalent) Compounds-Section 4.11 Answers
L. ____ (02) Bond Angles/Bond Lengths-Section 4.8 Answers
N. ____ (02) Geometry of Molecules-Section 4.8 Answers
O. ____ (02) Polarity of Molecules-Section 4.10 Answers
H. _____(03) Inorganic Compounds Sections 3.7, 3.8,3.9, 3.10, 3.11, 4.11 Answers
_______(15) Chapter 4 Total
Chapter
4.
Molecular Compounds Table of Contents
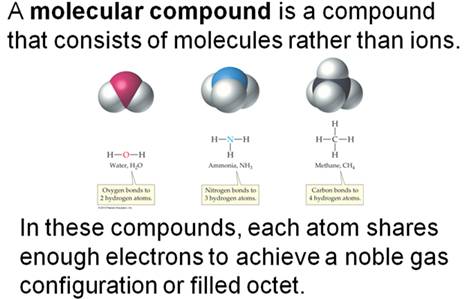
4.1 Covalent Bonds
4.2 Covalent Bonds and the Periodic Table
4.3 Multiple Covalent Bonds
4.4 Coordinate Covalent Bonds
4.5 Characteristics of Molecular Compounds
4.6 Molecular Formulas and Lewis Structures
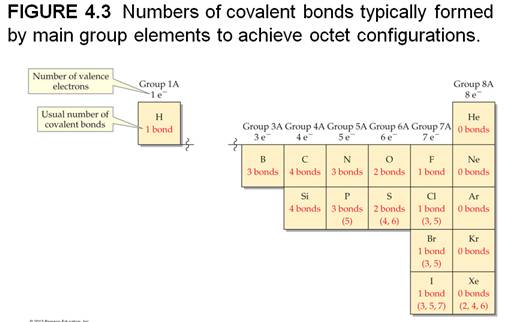
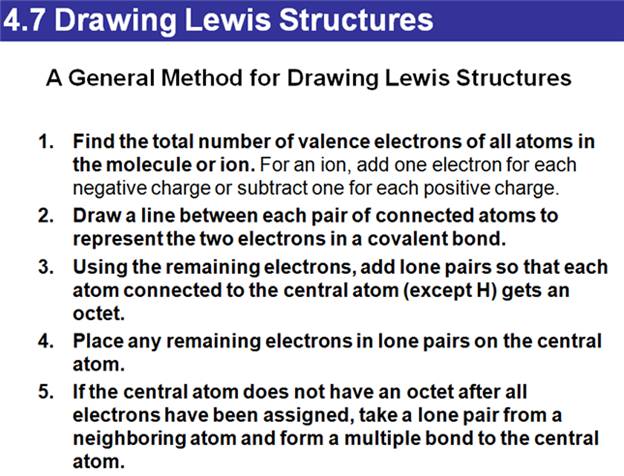
4.7 Drawing Lewis Structures
4.8 The Shapes of Molecules
4.9 Polar Covalent Bonds and Electronegativity
4.10 Polar Molecules
4.11 Naming Binary Molecular Compounds
See lab
handout and McMurry GOB Section 4.7 (or Corwin Chapter 12 sections 12.4-12.5
for directions) or (Hein Chapter 11 Sections 11.7, 11.8, 11.9)
Submit the following dot structures as a
separate Post Lab Report 10 points
Drag and Drop Interactive Web Site (Nothing required to turn in):
Check answers:
http://www.lsua.us/chem1001/dragdrop/menu.html
Module
Four: Part B Dot Structures of Molecules 4 points
Using
a periodic chart draw the electron dot structures of the following molecules:
(Choose One for each
question or the one circled on the paper)
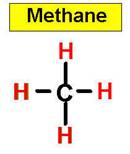
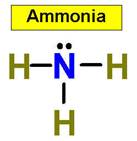
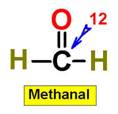
1. NH3 CH4 H2O2 H2O 2.
H2SO4 H3PO4 HClO4 HClO3
Submit these dot
structures as a separate homework
3. HNO3 H2CO3 HNO2 4.
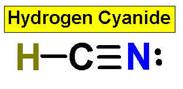
CO2 HCN SO3 SO2
Submit these dot
structures as a separate homework
5. HC2H3O2 H2C2O4 HCHO2
6.
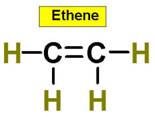
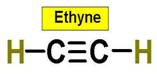
C2H4 C2H2 C3H8 C2H6
carbon to carbon by single
covalent bond bond
carbons to carbon
Submit these dot
structures as a separate homework
7. CH3CH2OH CH3COCH3 CH2O (HCHO)
(carbon to
carbons by single covalent bonds-oxygen attach to carbon)
Submit these dot
structures as a separate homework
8. CH3OCH3 CHONH2 CH3CH2CH2OH CH3CHOHCH3
oxygen
separates the carbons O & N
both bond to C (all three
carbons single bonded and OH attached to carbon)
Submit these dot
structures as a separate homework
9. CH2NH2COOH CH3CHNH2COOH
carbon to carbons by
single covalent bonds (-NH2 amino on#2 carbon in both above)
Submit these dot
structures as a separate homework
10. CH3COOCH2CH3 HCOOCH3
(-CH2CH3 also
hooks to oxygen in#10, as well as - CH3 )
Binary Molecular (Covalent)
Compounds-Section 4.11
Binary Molecular compounds are
explained after the ionic compounds in Chapter 4 GOB McMurry Section 4.11
(Corwin (7th) Chapter 6 section 6.7, and inorganic acids are not
covered until last in the chapter, sections 6.8 and 6.9) ( Hein includes Binary
Molecular at the end of section 6.4 [Page 108 14th] covering all
Binary Compounds first ionic, then molecular).
The required Online Binary Covalent Molecular Homework 2 points
each
The
web site is:
C: Binary Molecular Names:
http://www.northcampus.net/Nomenclature/Molecules/32BinaryCovalent.html
C1:
Binary Molecular Formulas:
http://www.northcampus.net/Nomenclature/MoleculeFormula/32BinaryMolecularFormula.html
Here is a brief tutorial for Part
C:
PART C: BINARY COVALENT COMPOUNDS
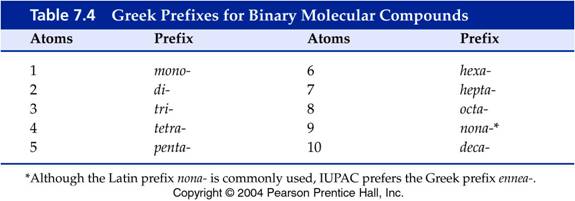
Both elements are nonmetals attached by covalent bonds. These bonds may be single, double, or triple covalent. Due to the covalent bonding there are many ratios of the same two elements making many different compounds. For this reason, the chemist states how many atoms of each element is present in the chemical formula in the formal name of the compound.
Prefixes are attached to each element to indicate how many. Each student should learn the following prefixes:
MONO
=
ONE
HEXA
= SIX
DI
=
TWO
HEPTA
= SEVEN
TRI
= THREE OCTA
= EIGHT
TETRA
=
FOUR
NONA
= NINE
PENTA
=
FIVE
DECA
= TEN
The element that is shown first in the chemical formula is written first using the proper prefix to indicate how may atoms of that element is contained in the compound. If there is only one atom of that element it is often found without the prefix mono. If you leave the prefix off then it is understood that you mean mono.
The element which is written second in the chemical formula is written second in the chemical name, but in addition to the prefix indicating how many, the suffix of the elements name is changed to -ide.
carbon becomes carbide chlorine becomes chloride
sulfur becomes sulfide oxygen becomes oxide
hydrogen becomes hydride nitrogen becomes nitride
Therefore, the following formulas of binary compounds would be spoken:
CCl4 carbon tetrachloride
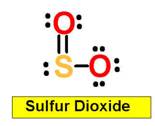
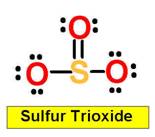
SO2 sulfur dioxide
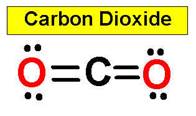
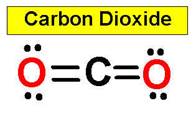
CO2 carbon dioxide
N2O3 dinitrogen trioxide
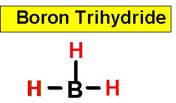
BH3 boron
trihydride
We use common names for NH3, and H2O. What would be their correct binary molecular names? Methane, CH4, is the organic name for CH4, what would its inorganic name be?
Chapter 4: Part
C Binary Molecular Compounds 2 points
Using
a periodic chart write the names or formulas of the following compounds
depending on whether the formula or name is given:
Homework
Packet Sample test: answer on grading outline
1. CO
____________________
2. SO3 _____________________
3. N2O5 _____________________
4. N2O7 _____________________
5. N2O _____________________
6. Phosphorus pentachloride _________
7. Boron trifluoride _________
8. Carbon dioxide _________
9. Sulfur Trioxide _________
10. Carbon Tetrachloride _________
Textbook Reference: McMurry Section 4.11 (Corwin Chapter
6 Section 6.7) (Hein
Section 6.4 page
108)
Online
Homework (2 Points Each Required):
C:
Binary Molecular (Covalent) Homework: http://www.northcampus.net/Nomenclature/Molecules/25BinaryCovalent.html
C1.
Binary Molecular (Covalent) Formulas: http://www.northcampus.net/Nomenclature/MoleculeFormula/25BinaryMolecularFormula.html
Submit
grades on separate grading Sheet (goldenrod) when taking Chapter 4 Exam
Online
Study Guide:
http://www.fccj.us/chm1025/AssignmentOutline/M4PartC.htm
Chapter
4 Part L: VSEPR and Bond Angles
Reference:
VSEPR Video:
http://www.lsua.info/chem1001/VSEPR/VSEPRtheory.wmv
What is VSEPR?
VSEPR
stands for Valence Shell Electron Pair Repulsion. It's a complicated
acronym, but it means something that's not difficult to understand.
Basically, the idea is that covalent bonds and lone pair electrons like to stay
as far apart from each other as possible under all conditions. This is
because covalent bonds consist of electrons, and electrons don't like to hang
around next to each other much because they have the same charge.
This
VSEPR thing explains why molecules have their shapes. If carbon has four
atoms stuck to it (as in methane), these four atoms want to get as far away
from each other as they can. This isn't because the atoms necessarily
hate each other, it's because the electrons in the bonds hate each other.
That's the idea behind VSEPR.
What
is a Bond Angle?
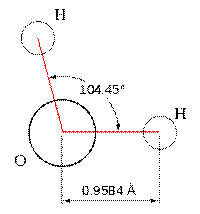
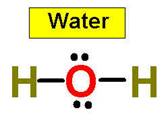
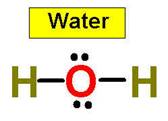
Lets look at the water molecule:
VSEPR table
The bond angles in the table below are ideal angles from the
simple VSEPR
theory, followed by the actual angle for the example given in the
following column where this differs. For many cases, such as trigonal pyramidal
and bent, the actual angle for the example differs from the
ideal angle, but all examples differ by different amounts. For example,
the angle in H2S (92°) differs from the tetrahedral angle by much
more than the angle for H2O (104.5°) does.
|
Bonding
electron pairs CHM 1032C |
Lone
pairs |
Electron
domains (Steric #) |
Shape |
Ideal
bond angle (example's bond angle) |
Example |
Image |
|
2 |
0 |
2 |
180° |
|||
|
3 |
0 |
3 |
120° |
|||
|
2 |
1 |
3 |
120° (119°) |
|||
|
4 |
0 |
4 |
109.5° |
|||
|
3 |
1 |
4 |
107° |
|||
|
2 |
2 |
4 |
bent |
109.5° (104.5°) |
||
|
5 |
0 |
5 |
90°, 120°, 180° |
|||
|
4 |
1 |
5 |
180°, 120°, 90° (173.1°, 101.6°) |
|||
|
3 |
2 |
5 |
90°, 180° (87.5°, < 180°) |
|||
|
2 |
3 |
5 |
linear |
180° |
||
|
6 |
0 |
6 |
90°, 180° |
|||
|
5 |
1 |
6 |
90° (84.8°), 180° |
|||
|
4 |
2 |
6 |
90°, 180° |
|||
|
7 |
0 |
r7r |
90°, 72°, 180° |
|||
|
6 |
1 |
7 |
72°, 90°, 144° |
XeOF5− |
||
|
5 |
2 |
7 |
72°, 144° |
|||
|
8 |
0 |
8 |
||||
|
9 |
0 |
9 |
|
Example Predict
all bond angles in the following molecules. a.
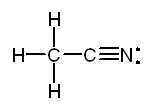
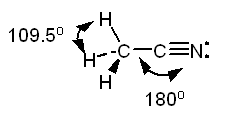
CH3Cl b. CH3CNl
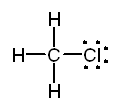
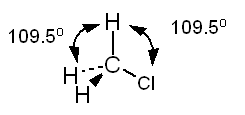
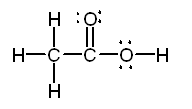
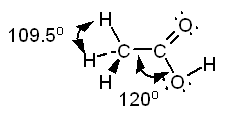
c. CH3COOH Solution
a.
The Lewis structure of methyl chloride is:
In
the Lewis structure of CH3Cl carbon is surrounded by four regions
of high electron density, each of which forms a single bond. Based on the
VSEPR model, we predict a tetrahedral distribution of electron clouds around
carbon, H - C - H and H - C - Cl bond angles of 109.5°, and a tetrahedral
shape for the molecule. Note the use of doted lines to represent a bond
projecting behind the plane of the paper and a solid wedge to represent a
bond projecting forward from the plane of the paper.
b.
The Lewis structure of acetonitrile, CH3CN is:
The
methyl group, CH3-, is tetrahedral. The carbon of the -CN group is
in the middle of a straight line stretching from the carbon of the methyl
group through the nitrogen.
c.
The Lewis structure of acetic acid is:
Both
the carbon bonded to three hydrogens and the oxygen bonded to carbon and
hydrogen are centers of tetrahedral structures. The
central carbon will have 120 7deg bond angles.
The
geometry around the first carbon is tetrahedral, around the second carbon
atom is trigonal planar, and around the oxygen is bent. |
Chapter
4: Part L Bond Angles 02 points
What is the bond Angle in the following structures:
___1. 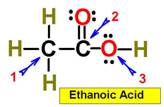 ___4.
___4.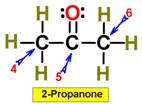
___2. ___5.
___3. ___6.
___7. 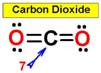 ___8.
___8.  ____9.
____9.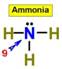
___10. 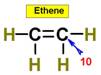 ____11.
____11. 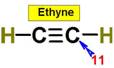
___12. 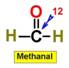 ___13.
___13. 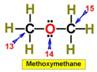 ___16.
___16.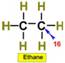
___14.
___15.
___17.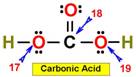 ___20.
___20. 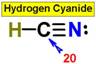
Bonus:
___21. 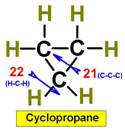 ___23.
___23. 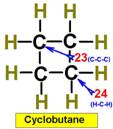
___22. ___24.
Steric Numbers do not predict bond angles within
rings of carbons
Types of molecular structure
Some common shapes of simple
molecules include:
- Linear: In a linear model, atoms
are connected in a straight line. The bond angles are set at 180°. A bond
angle is very simply the geometric angle between two adjacent bonds. For
example, carbon dioxide and nitric oxide have a linear molecular
shape.
- Trigonal planar: Just from its name, it can
easily be said that molecules with the trigonal planar shape are somewhat
triangular and in one plane
(flat).
Consequently, the bond angles are set at 120°. An example of this is boron
trifluoride.
- Bent: Bent or angular molecules
have a non-linear shape. A good example is water, or H2O, which
has an angle of about 105°. A water molecule has two pairs of bonded
electrons and two unshared lone pairs.
- Tetrahedral: Tetra- signifies four,
and -hedral relates to a face of a solid, so "tetrahedral" literally means
"having four faces". This shape is found when there are four
bonds all on one central atom, with no extra unshared electron pairs. In accordance with
the VSEPR (valence-shell electron
pair repulsion theory), the bond angles between the electron bonds are arccos(−1/3) = 109.47°.
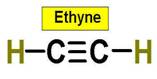
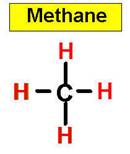
An example of a tetrahedral molecule is methane (CH4).
- Octahedral: Octa- signifies
eight, and -hedral relates to a face of a solid, so "octahedral" literally means
"having eight faces". The bond angle is 90 degrees. An
example of an octahedral molecule is sulfur
hexafluoride
(SF6).
- Pyramidal:
Pyramidal-shaped molecules have pyramid-like
shapes. Unlike the linear and trigonal planar shapes but similar to
the tetrahedral orientation, pyramidal shapes
require three dimensions in order to fully separate the electrons. Here,
there are only three pairs of bonded electrons, leaving one unshared lone
pair. Lone pair bond pair repulsions change the angle from the
tetrahedral angle to a slightly lower[citation needed]
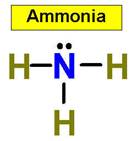
value. An example is NH3 (ammonia).
CHM 1032C Common shapes you should know
There are a whole bunch of common shapes you need to know to accurately
think of covalent molecules. Here they are:
- Tetrahedral: Tetrahedral molecules look like pyramids
with four faces. Each point on the pyramid corresponds to an atom
that's attached to the central atom. Bond angles are 109.5 degrees.
- Trigonal pyramidal: It's like a tetrahedral molecule,
except flatter. It looks kind of like a squished pyramid because one
of the atoms in the pyramid is replaced with a lone pair. Bond
angles are 107.5 degrees (it's less than tetrahedral molecules because the
lone pair shoves the other atoms closer to each other).
- Trigonal planar: It looks like the hood ornament of a
Mercedes automobile, or like a peace sign with that bottom-most line
gone. The bond angles are 120 degrees.
- Bent: They look, well, bent. Bond angles can be either 118
degrees for molcules with one lone pair or 104.5 degrees for molecules
with two lone pairs.
- Linear: The atoms in the molecule are in
a straight line. This can be either because there are only two atoms
in the molecule (in which case there is no bond angle, as there need to be
three atoms to get a bond angle) or because the three atoms are lined up
in a straight line (corresponding to a 180 degree bond angle).
- There are other types, but we won't
worry about them.
Trigonal-bipyramidal Square Planer Seesaw T-shaped Octahedral
Module Four- Part N: Geometry of Molecules 2 points
Use the dot/stick structures on the Part L page to state the geometry of the molecules:
Bent Linear Trigonal Planer Planer Trigonal Pyramidal Tetrahedral
Trigonal-bipyramidal Square Planer Seesaw T-shaped Octahedral
|
_____________1.
H2O _____________2.
CO2 _____________3.
C2H4 _____________4.
SO2 _____________5.
SO3 _____________6.
HCN _____________7.
CH4 _____________8.
NH3 _____________9.
CH2O _____________10. C2H2 _____________Bonus. PF5
_____________Bonus SF6 |
|
Polar
Covalent Bonds
Covalent bonds result from the sharing of valence electrons.
Often, the two atoms do not share the electrons equally. That is, one of
the atoms holds onto the electrons more tightly than the other.
When one of the atoms holds the shared electrons more tightly, the bond
is polarized.
A
polar covalent bond is one in which the electrons are not shared equally
Electronegativity
Each
element has an innate ability to attract valence electrons.
Electronegativity is the ability of an atom to
attract electrons in a chemical bond.
Linus
Pauling devised a method for measuring the electronegativity of each of the
elements.
Fluorine
is the most electronegative element.
Electronegativity
increases as you go left to right across a period.
Electronegativity
increases as you go from bottom to top in a family.
Electronegativity: The ability of an atom in a
molecule to attract the shared electrons in a covalent bond.
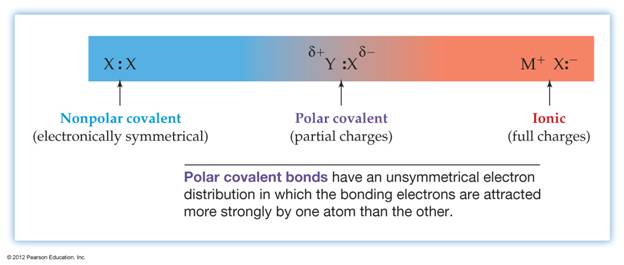
Electronegativity Differences

The
electronegativity of H is 2.1; Cl is 3.0.
Since
there is a difference in electronegativity between the two elements (3.0 2.1
= 0.9), the bond in H Cl is polar.
Since
Cl is more electronegative, the bonding electrons are attracted toward the Cl
atom and away from the H atom. This will give the Cl atom a slightly negative
charge and the H atom a slightly positive charge.
Nonpolar Covalent Bonds
What
if the two atoms in a covalent bond have the same or similar electronegativities?
The
bond is not polarized and it is a nonpolar covalent bond.
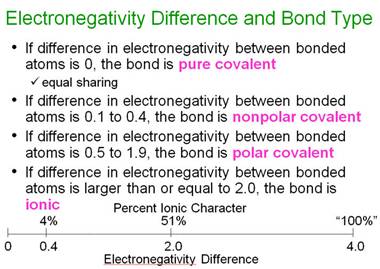
If the electronegativity difference is less than 0.5, it is usually considered
a nonpolar bond.
The
diatomic halogen molecules have nonpolar covalent bonds.
Chapter 4- Part O: Polarity of Molecules 2 points
Use the dot/stick structures and sketch the molecule in three dimensions. Then draw the dipoles for each bond to state if the molecule is polar or nonpolar:
Electronegativities: F=4.0; O=3.5; N=3.0; Cl=3.0; Br=2.7; C=2.5; S=2.5;
P=2.1; H=2.1
|
_____________1. H2O _____________2. CO2 _____________3. C2H4 _____________4. C2H2
_____________5. SO2
_____________6. SO3
_____________7. CH4 _____________8. NH3 _____________9. BH3 _____________10. HCN
_____________Bonus. PCl5 _____________Bonus SCl6 |
|
Chapter 4: Part H Inorganic Compounds 3 points
The key to deciding which system to use in Part H is to look at the element written first.
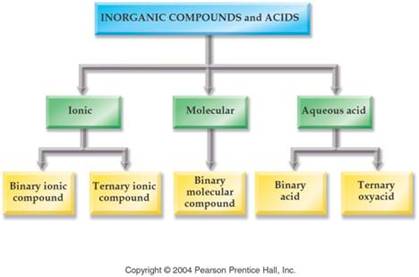
1. If a Metal is written first (or a polyatomic ion), then use the rules for ionic compounds (salts).
2. If a nonmetal is written first, then use the Covalent/Molecule System with prefixes. (If the compound is Organic Nomenclature of Organics is covered in Chapter 11, but for now use the prefix system of binary molecular nomenclature.
3. If hydrogen
is written first (and it is in aqueous solution) then name it as an Acid
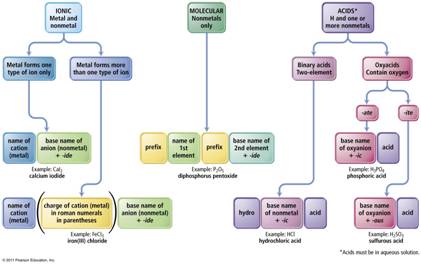
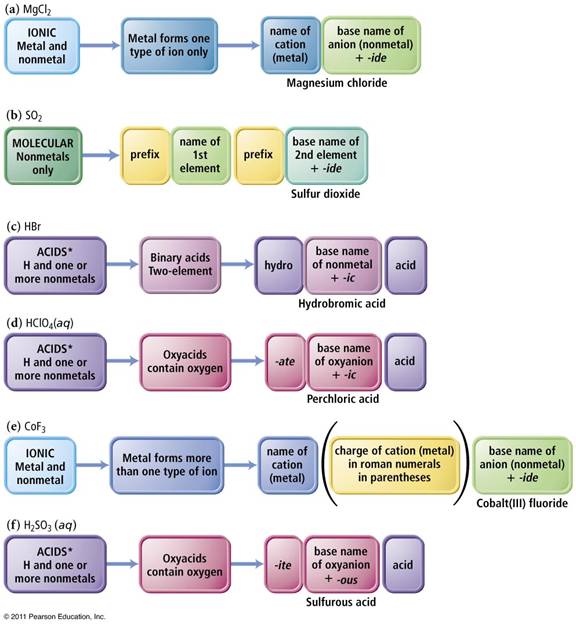
Chapter 4: Part H Inorganic Compounds 3 points
Using
a periodic chart write the names or formulas of the following polyatomic ions
depending on whether the formula or name is given:
1. H2CO3 _____________________
2. MgSO4 _____________________
3. Ca3(PO3)2 ____________________
4. HClO3 _____________________
5. SO3 _____________________
6. Fe2O3 _____________________
7. Aluminum Hydroxide ____________
8. Ammonium chloride _____________
9. Sodium Hypochlorite _____________
10. Nitrogen dioxide _____________
11. Calcium Phosphate ____________
12. Sulfuric acid ____________
Online
Homework (2 Points Each Required):
H:
Inorganic Compound Names Homework: http://www.northcampus.net/Nomenclature/Inorganic/32inorganic.html
H1.
Inorganic Compound Formulas:
http://www.northcampus.net/Nomenclature/InorganicFormula/25inorganicFormula.html
Submit
grades on separate grading Sheet when taking Chapter-4 Exam
Online Study Guide:
http://www.fccj.us/chm1025/AssignmentOutline/M4PartH.htm