CHM1032C Grading Outline
Chapter 6 Chemical Reactions:
Moles and Mass Relationships
A._____(02) Molecular Mass Calculation-Section6.1 Answers a
B._____(02) Mole
Calculations I-Sections 6.2 Answers
bcd
B1._____(02) Mole Calculations
II-Sections 6.2 Answers
bcd
C._____(02) Percentage Composition
Calculation-Lecture Answers
bcd
D._____(02) Empirical Formula
Calc. from % Comp-Lecture Answers
bcd
D1._____(02) Empirical Formula
Calc. from Lab Data-Lecture Answers bcd
I.______(02) Mole-Mole Problems Section 6.3 Answers ij
J._____ (03) Mass-Mass Stoichiometric Problems-Section 6.4 Answers ij
K._____(03) Excess/Limiting Reagent ProblemsSection 6.5 -Answers kl
L._____(02) Percent Yield-Section
6.5 Answers
______(14) Chapter 6 Total
Chapter 6: Chemical Reactions:
Mole and Mass Relationships Table Contents
6.1 The Mole and Avogadro’s Number M-5B
6.2 Gram—Mole ConversionsM-5A/B1
6.3 Mole Relationships and Chemical Equations M-5I
6.4 Mass Relationships and Chemical EquationsM-5J
6.5 Limiting Reagent and Percent Yield M-5K
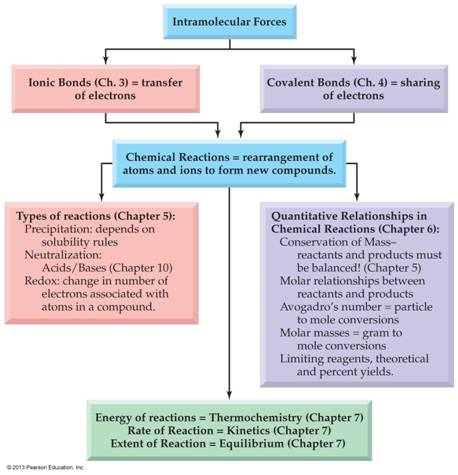
Chapters
3 through 7 Concept Map:
Module
Five-Part A: Molecular Mass Calculation 2 points
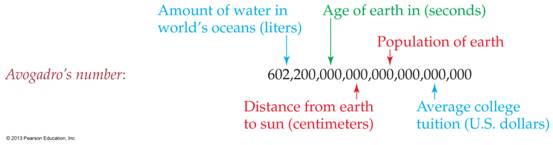
1 mole of atoms = 6.023 x 1023 atoms. See “What is a mole?” Lab
Analogies.
The atomic mass of any substance expressed in grams is the molar mass
(MM) of that substance.
•
The
atomic mass of carbon is 12.01 amu per atom.
•
Therefore,
the molar mass of carbon is 12.01 g/mol .
•
Since
nitrogen occurs naturally as a diatomic, N2, the molar mass of
nitrogen gas is two times 14.01
g or 28.02 g/mol.
Calculating
Molar Mass
•
The
molar mass of a substance is the sum of the molar masses of each element.
•
What
is the molar mass of copper(II) nitrite, Cu(NO2)2?
•
The
sum of the atomic masses is as follows:
63.55 + 2(14.01 + 16.00 +
16.00) =
63.55 + 2(46.01) = 155.57 amu per molecule
•
The
molar mass for Cu(NO2)2 is
155.57 g/mol.
Molar Mass Connects Moles to Grams & Vice versa:
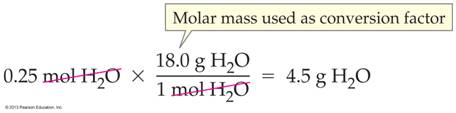
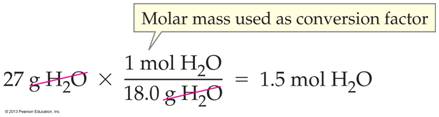
Module
Five-Part A: Molecular Mass Calculation 2 points
Homework #1:
Using a periodic
chart calculate the molar mass of the following:
1. Calculate the molecular
mass of Acetic Acid, HC2H3O2.
2. Calculate the
formula unit mass of Ammonium Chromate,
(NH4)2CrO4 .
3. Calculate the
molecular mass of glucose,
C6H12O6.
Reference:
McMurry Section 6.2 Try problems: 6.23,
6.24, 6.27, 6.30, 6.31, 6.32, 6.33, 6.34, 6.35
Corwin
Section 8.3 Additional Problems: Corwin #13-#16 Pages 244-245
Hein:
Section 7.2: Example 7.7; 7.8 End of Chapter #1-#2 Page 139
Interactive Online Chem-i-Calc(Molar Mass & %
Composition):
http://people.emich.edu/bramsay1/ccc-release/chem.html
Chapter
6 Sections 6.3-6.4-Lecture
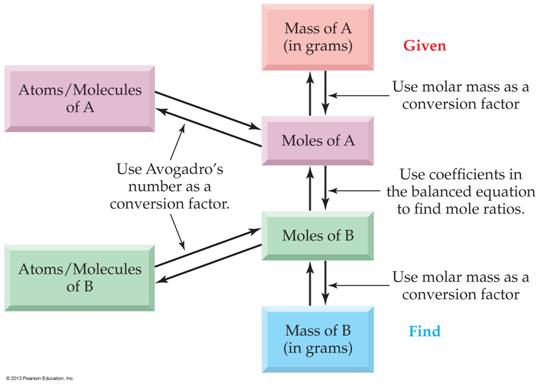
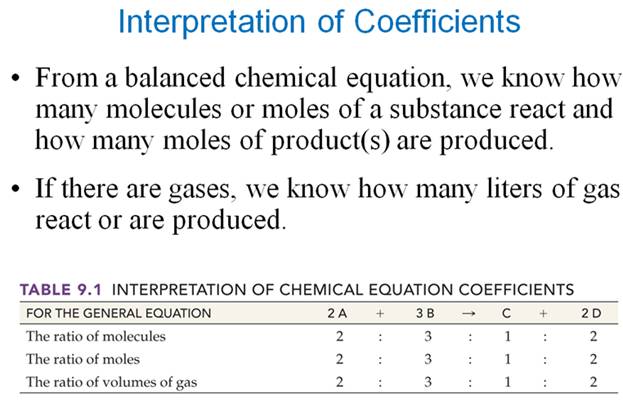
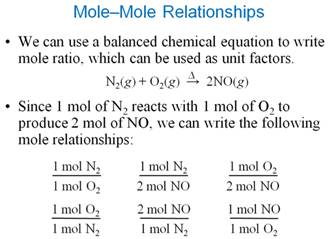

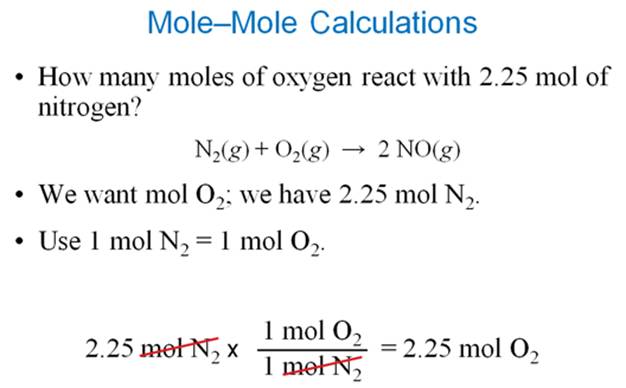
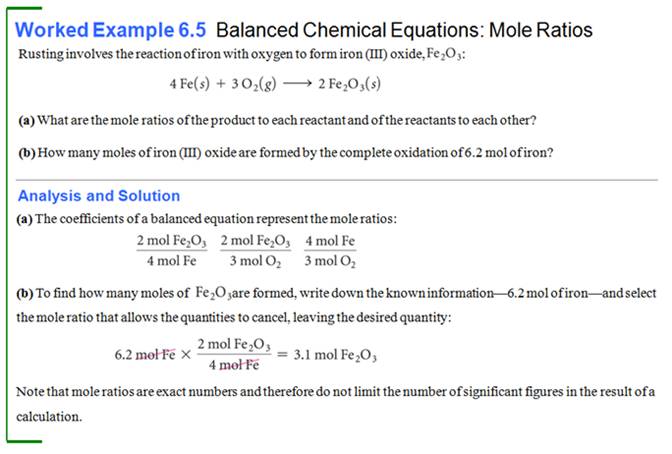
From Another Text:
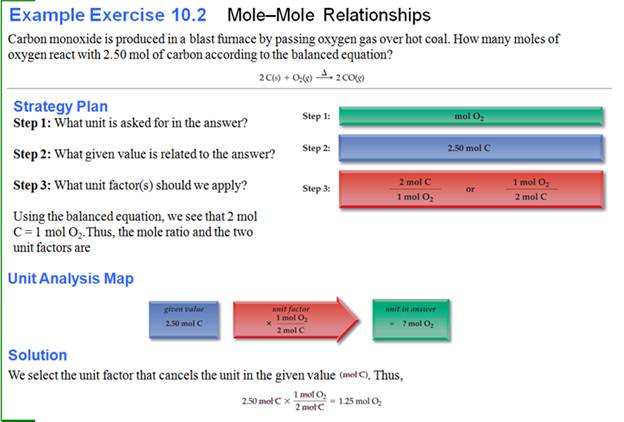
Chapter 6: Part
I Mole-Mole
Stoichiometry 2 points
Homework #1: Tungsten occurs in the important mineral sheelite (Calcium tungstate),
which is converted to tungstic acid. Tungsten is then extracted from tungstic acid by the following (unbalanced) reaction:
H2
+ H2WO4 à W +
H2O (Unbalanced)
How moles of hydrogen is needed to prepare 6 moles of elemental
tungsten?
Homework #2: Phosphoric acid can be made by the following (unbalanced) reaction:
H2O + P4O10 à H3PO4 (unbalanced)
How many moles of
Phosphoric acid can be prepared from the combination of 5 moles of Tetraphosphorus decoxide with
excess water?
References:
See
McMurry: Section 6.3-6.4; See Worked Example 6.5; Try Problem6.8 page 166
Try
End of Chapter: Problems 6.39, 6.40
See
Hein Worked Examples: 9.2-9.5 pages
170-173
Try Practice 9.2 and 9.3 page 173
Also try Problems 9-10-11-12 page 184
Corwin
7th Reference: Section 9.1-9.2
Corwin 1025: see worked Examples 9.2 page 253
additional Suggested Problems: Page 273-4 #7-#12
Chapter
6 Section J: Mass-Mass Stoichiometry 3 Points
Use this concept map for Part J Mass-Mass Problems:
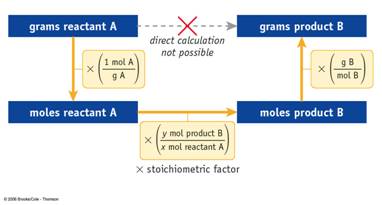
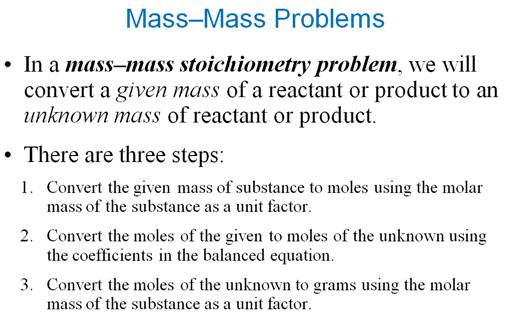
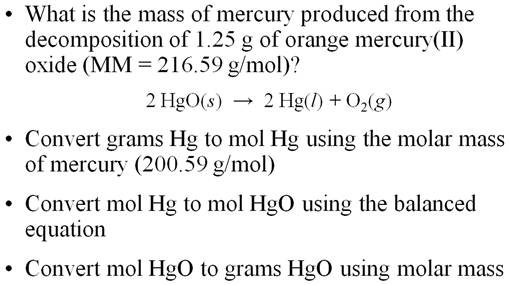
Step 1:
A Mass-Mass Worked Example From Another
book:
Using all three steps:
The Solution to: __?____g Hg = 1.25g 1.25 g HgO
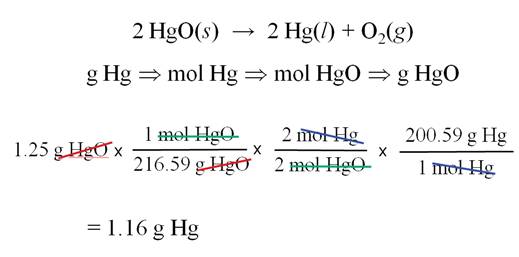
Another Mass-Mass Worked Example from
Another book
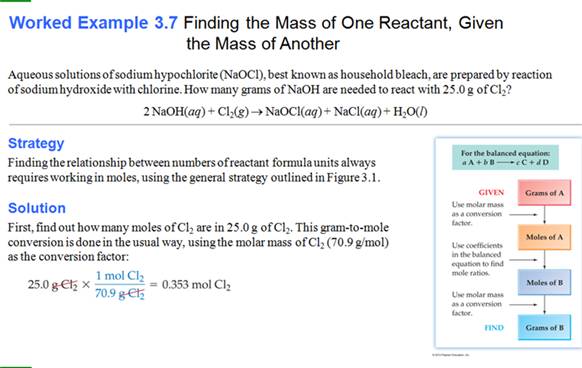
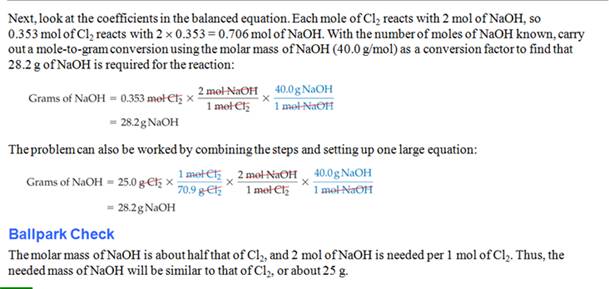
Still Another Worked Example from Another
book:
From book to book, the three steps are illustrated:
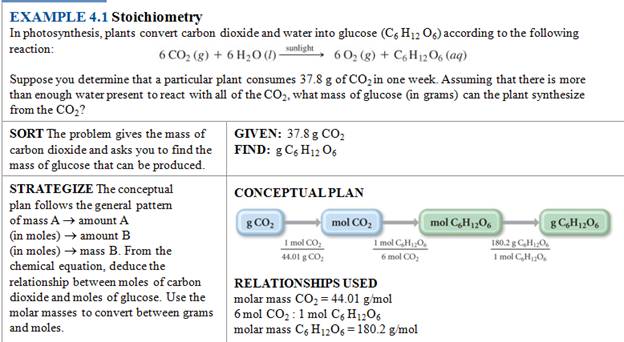
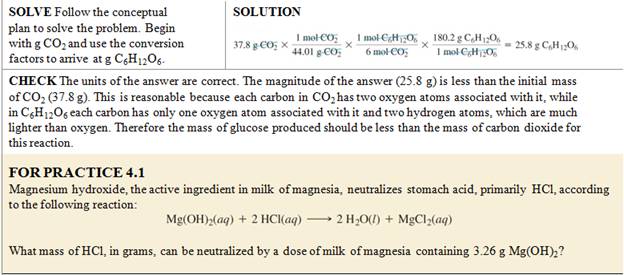
Chapter
6 Section J: Mass-Mass Stoichiometry 3 Points
Homework
#3: Toluene and nitric acid
are used in the production of trinitrotoluene (TNT), an explosive:
C7H8 +
HNO3 à C7H5N3O6 +
H2O (Unbalanced)
Calculate the mass
of TNT that can be made from 192 g of C7H8 (toluene).
(You must use dimensional analysis to
show your work!)
Homework
#4: What
mass of carbon dioxide is produced from the combustion of 176 grams of propane gas , C3H8
, in excess oxygen gas, O2.
Water is the only other product.
(You must use
dimensional analysis to show your work!)
Write the Balanced Reaction:
Homework
#5:
- What mass of carbon
dioxide is produced from the combustion of 456 grams of gasoline
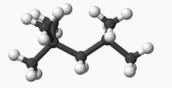
, C8H18
, (2,2,4 Trimethyl Pentane)in excess oxygen
gas, O2. Water is the only other product.
(You must use dimensional analysis to show your work!)
Write the Balanced Reaction:
Engine
knocking is an unwanted process that can occur during combustion in internal combustion engines. Graham Edgar in 1926 added
different amounts of n-heptane C7H14)and
2,2,4-trimethylpentane to gasoline, and discovered that the knocking stopped
when 2,2,4-trimethylpentane was added. This was the origin of the octane
rating scale.[5]
Test motors, using 2,2,4-trimethylpentane gave a
certain performance which was standardized as 100 octane. The same test motors,
run in the same fashion, using heptane, gave a
performance which was standardized as 0 octane. All
other compounds and blends of compounds then were graded against these two
standards and assigned octane numbers.
|
Gasoline Molecule
|
|
- Prove the
quotation using dimensional analysis that burning one gallon of gasoline
releases 18.7 tons of Carbon dioxide to the environment in a gasoline
powered automobile. (Hint: There are 4 quarts = 1 gallon; 946 mL = 1 quart;
454 grams = 1 pound; 2000 pounds = 1 ton)
- How many
grams of oxygen gas will be the minimum requirement to completely combust
the 456 grams of gasoline (just over a pound)?
- How many
grams of water gas will exhaust out the tail pipe when the 456 grams of
gasoline is combusted?
References:
McMurry:
See Worked examples 6.6 and 6.7 page 168Try Problem 6.10 page 169.
Also try end of chapter problems: 6.39 to 6.51 pages 175-176
See Hein Worked Examples 9.8-9.9 pages 174-175
Try Practice 9.6 and 9.7 page 176
Also try End of Capter Problems 13-18 page 184
see Corwin worked Examples 9.4 p256
and 9.5 p257
Try end of Chapter additional Suggested Corwin Problems: Page 274 #19-#27
Part K
Excess-Limiting Reagent Problem 3
points
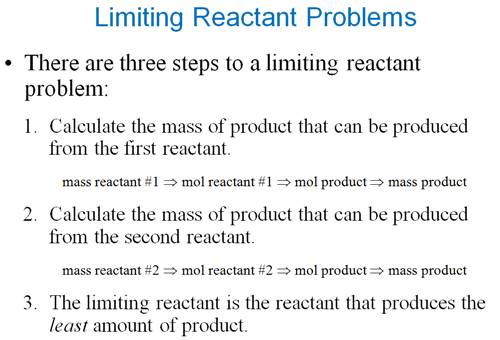
Sample Limiting Reagent Problem (Chapter
6 Part K)
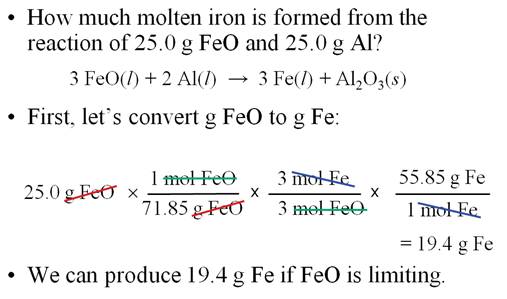
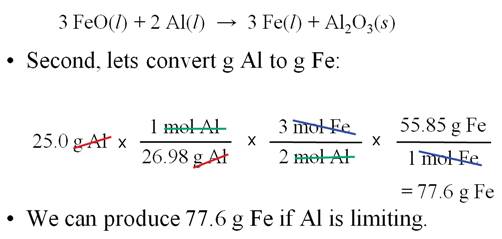

Some books teach you to determine
which reagent is the limit first, then do the standard
gram-gram problem
Either works, but I prefer the method above
(working two separate
gram-gram problems and which produces the lowest number that is the correct
sequence and answer.
Reference:
McMurry: Chapter 6 Section 6.5
See Worked Examples
6.9, 6.10, and 6.11 which also includes Percent yield (Chapter 6 Part L) pages
170-171; Try Problems 6.12, 6.13, and 6.14 page 171
Corwin: Review Sections 9.7 and 9.8
see worked Examples 9.10 p267
additional Suggested Problems: p276 #59-#74
Hein: Chapter 9 Section 9.5
see worked examples 9.11, 9.12, 9.13, 9.14 pages 178-181
Try problem 9.9 page 180; 9.10 page 181
End of chapter: Try