CHM
2045C Module 1 Homework Packet Name:___________________
Module One: Matter and States of Matter (Chapter
1) ppt
A. _____(05) Matter Chart-Section
2.10 Figure 2.10 p54 Answers
A1._____(05) Critical Thinking
Matter Chart Applications Answers
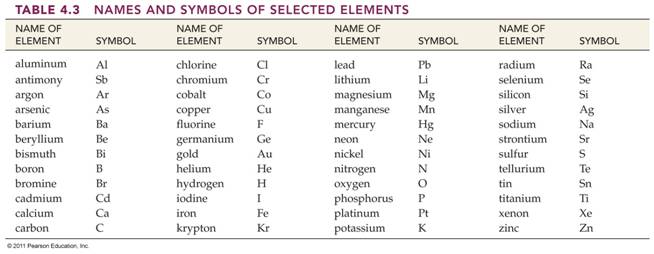
B. _____(15) Element-Symbol-Section
1.2 Table 1.1 + Answers Element List
B1._____(05) Element Identification
(Click on element on chart)
C. _____(05) Element Classification-Section
1.4 page 9 Answers ck
C1.____ (05) Compounds and
Chemical Formulas Answers
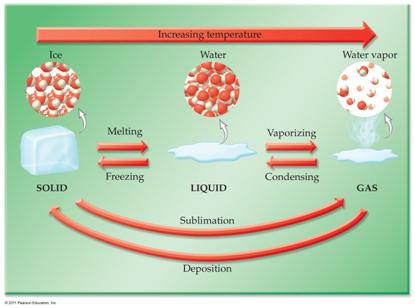
F. _____(10) Phase Diagram
Section 10.11 Answers Real Video Movie
_______(50) Total = ______%
M. _____(50) Multiple Choice(Matter)
Chap 1 Online Blackboard Homework
M-1 Required Homework:
B1.______(20) Online/Flash Cards Element Homework
______(50) M-1
Pretest Hardcopy Homework Packet
______(50) M-1 Multiple Choice (MC)
Practice (Blackboard Online)
)
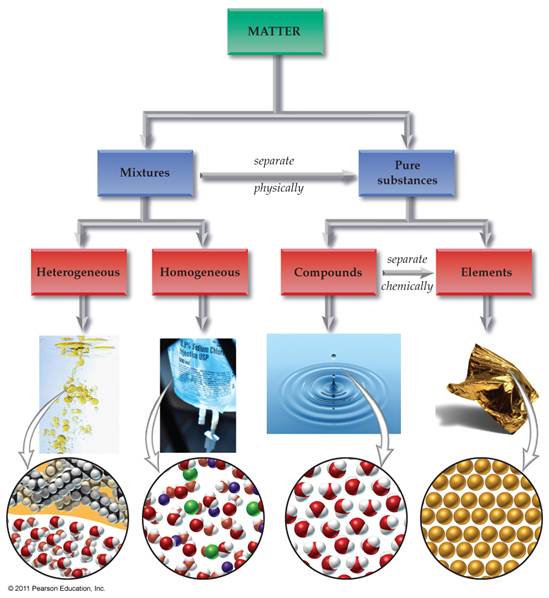
Module One: Part A Matter Chart 05
points
Draw below an
expanded matter chart similar to the chart shown on our web site. The chart should include the following: homogeneous mixtures, heterogeneous
mixtures, Matter, Pure Substances, Mixtures, Compounds, Elements, Solutions,
Atoms, Molecules/Formula Units, Colloids, and Suspensions. Also draw/label the arrows: Separate
Physically and Separate Chemically:
Matter Chart Homework Critical Thinking 05 Points:
How would you show:
metals, nonmetals, metalloids, inorganic compounds, organic compounds,
colloids, electrons, protons, neutrons, nucleus, orbitals. Sketch you suggestions below:
If you put: Inorganic Compounds and Organic Compounds
under compounds., how would you split Molecules
and Formula units then show Salts, Acids, Bases, Covalent Compounds under the
correct classification?
Module One
Homework Packet
CHM 2045C Module
One Homework Packet
M-1 Part B:
Element/Symbol 15 points
In the blanks below, write the symbol
for the element listed (1/2 pt each), or write the name (with correct spelling)(1 points each) for the element represented by its symbol:
1. Magnesium _____ 21. Cl __ ___________
2. Manganese _____ 22. F _____________
3. Tungsten _____ 23. I _____________
4. Platinum _____ 24. Br _____________
5. Gold _____ 25. Zn _____________
6. Silver _____ 26. H _____________
7. Iron _____ 27. O _____________
8. Tin _____ 28. N _____________
9. Helium _____ 29. C _____________
10.
Antimony _____ 30. K _____________
11.
Lead _____ 31. P _____________
12.
Argon _____ 32. B _____________
13. Neon _____ 33. Al _____________
14. Krypton _____ 34. Cr _____________
15. Arsenic _____ 35. Ca _____________
16. Mercury _____ 36. Bi _____________
17. Copper _____ 37. Sr _____________
18. Cobalt _____ 38. Si _____________
19. Beryllium _____ 39. Ni _____________
20. Selenium _____ 40. Li _____________
CHM 2045C Required Element List:
http://www.fccj.us/chm2045/Elements/45ElementTable.htm
CHM 2045C Online Element Spelling
Homework:
http://www.fscj.me/chm2045/elementquiz/elementquiz.html
CHM
2045C Module One: Element Identification Homework 05 points
Identify each of the following elements chalk board representation:

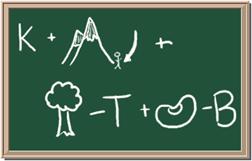
1. Element: _______________Symbol:_____ 2.Element: _______________Symbol:_____
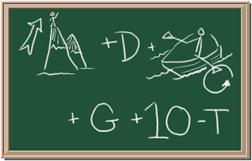
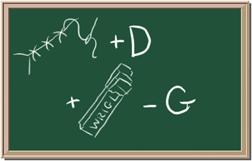
3. Element: _______________Symbol:_____ 4.Element: _______________Symbol:_____
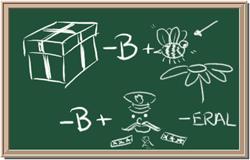

5. Element: _______________Symbol:_____ 6.Element: _______________Symbol:_____
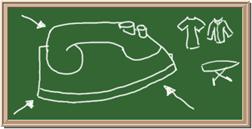
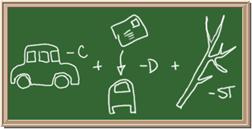
7. Element: _______________Symbol:_____ 8.Element: _______________Symbol:_____
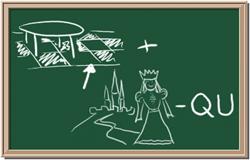
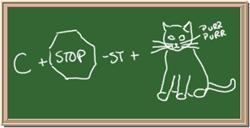
CHM 2045C Module One
Homework Packet
M-1 Part C: Element Classification 05 points
Using
the periodic chart provided, in the blanks below write the classification of
the elements listed:
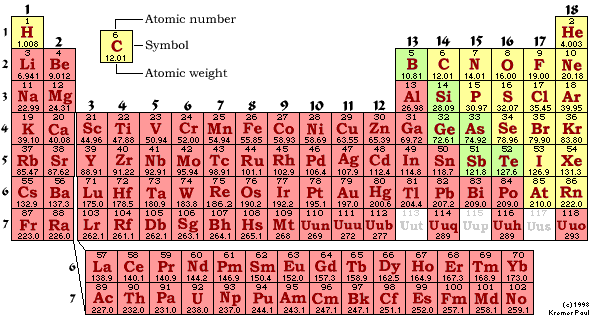
The
classifications of the elements are:
Metals;
Semimetals
(Metalloids);
Nonmetals-Regular;
Nonmetals-Nobel
Gases;
Nonmetals-Class
by Itself
Use
the above classifications to classify each of the following elements.
You
must split the nonmetals into three subclasses:
1. 12C6 ______________
2. 39K19 ______________
3. 11B5 ______________
4. 1H1 _________________
5. 4He2 _________________
6. 56Fe26 _________________
7. 238U92 _________________
8. 73Ge32 _________________
9. 9Be4 _________________
10. 86Rn222 _________________
CHM 2045C Module One Homework Packet
Part
C1: Compounds and Chemical Formulas 05 points
1.
State the Law of
Definite Composition:
2.
Define Molecule:
3.
The chemical
formula for vitamin B3 is: C6H6N2O
In One Molecule How Many:
_____ carbon
atoms
_____ oxygen
atoms
_____ total
atoms
4.
Write the
chemical formula for Vitamin B6 which has eight Carbon atoms, 11
Hydrogen atoms, one Nitrogen atom, and three Oxygen atoms:
How many
total atoms are there in one Vitamin B6 molecule?
5.
Citric Acid (in
citrus fruit) has the following chemical formula:
C3H4OH(COOH)3
In one
molecule of Citric Acid, How many:
______Carbon
atoms
______Oxygen
Atoms
______
Hydrogen Atoms
______ total atoms
CHM 2045C Module One Homework Packet
Part
F: Phase Diagrams 10 points
Identify the
points labeled on the
Phase Diagram of water:
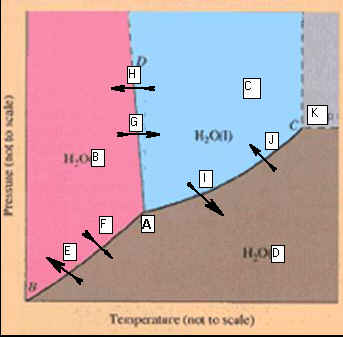
_____________________A.
_____________________B. _____________________H
_____________________C. _____________________I
_____________________D. _____________________J
_____________________E. _____________________K
_____________________F
_____________________G
CHM1025C Module One Homework Packet
Phase Diagram for Carbon Dioxide.
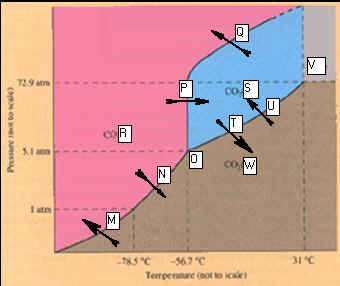
_____________________M.
____________________T
_____________________N. _____________________U
_____________________O. _____________________V
_____________________P. _____________________W
_____________________Q.
_____________________R
_____________________S