CHM
2045C Module 1 Homework Packet Name:___________________
(Jespersen
7th Edition)
Module One: Matter and States of Matter (Chapter
1) ppt
A. _____(01) Matter Chart-Section
2.10 Figure 2.10 p54 Answers
A1.____ (01) Critical Thinking
Matter Chart Applications Answers
B. _____(02) Element-Symbol-Section
1.2 Table 1.1 + Answers Element List
B1._____(01) Element Identification
(Click on element on chart)
C. _____(01) Element Classification-Section
1.4 page 9 Answers ck
C1.____ (01) Compounds and
Chemical Formulas Answers
F. _____(03) Phase Diagram
Section 10.11 Answers Real Video Movie
F1.____
(01) Phase Diagram Applications
_______(11) Total = ______%
M-1 Required Online Homework:
B1.______(04) Online/Flash Cards Element Homework (Unlimited Attempts)
(or Create Hard Copy Flash cards & show on M-1Exam Day 4
points)
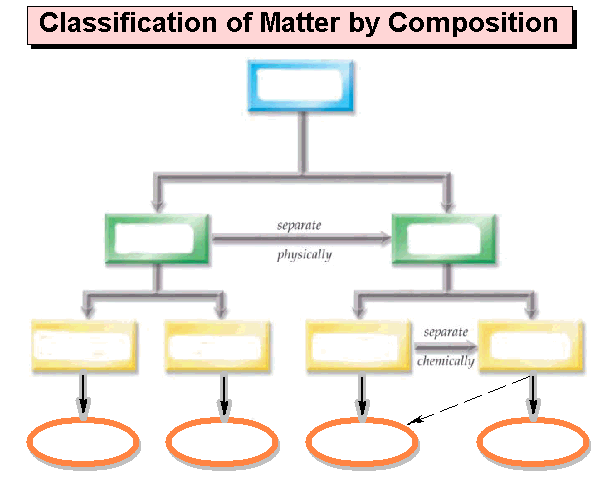
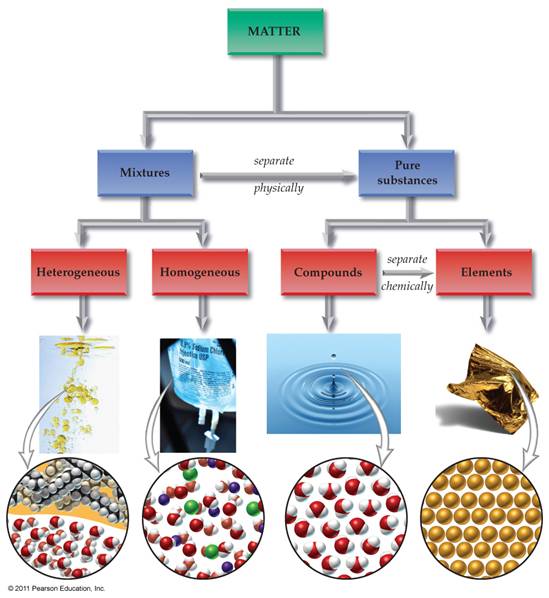
Module One: Part A Matter Chart 01 points
Draw below an
expanded matter chart similar to the chart shown on our web site. The chart should include the following: homogeneous mixtures, heterogeneous
mixtures, Matter, Pure Substances, Mixtures, Compounds, Elements, Solutions,
Atoms, Molecules/Formula Units, Colloids, and Suspensions. Also draw/label the arrows: Separate
Physically and Separate Chemically: Draw Chart Bellow:
Matter Chart Homework Critical Thinking 01 Points:
How would you show:
metals, nonmetals, metalloids, inorganic compounds, organic compounds,
colloids, electrons, protons, neutrons, nucleus, orbitals.
Sketch you suggestions below:
If you put: Inorganic Compounds and Organic Compounds
under compounds., how would you split
Molecules and Formula units then show Salts, Acids, Bases, Covalent Compounds
under the correct classification?
Fill in Chart Below:
Module One
Homework Packet
CHM 2045C Module
One Homework Packet
M-1 Part B:
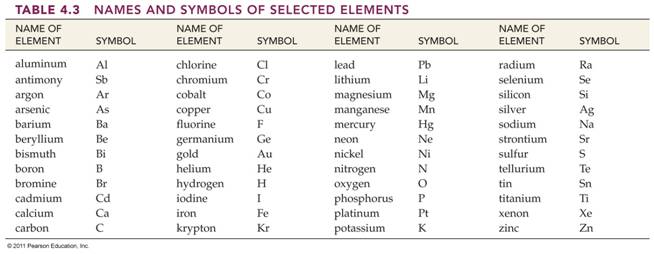
Element/Symbol 02 points
In the blanks below, write the symbol
for the element listed , or write the name (with
correct spelling) for the element represented by its symbol:
1. Magnesium _____ 21. Cl __
___________
2. Manganese _____ 22. F _____________
3. Tungsten _____ 23. I _____________
4. Platinum _____ 24. Br _____________
5. Gold _____ 25. Zn _____________
6. Silver _____ 26. H _____________
7. Iron _____ 27. O _____________
8. Tin _____ 28. N _____________
9. Helium _____ 29. C _____________
10.
Antimony _____ 30. K _____________
11.
Lead _____ 31. P _____________
12.
Argon _____ 32. B _____________
13. Neon _____ 33. Al _____________
14. Krypton _____ 34. Cr _____________
15. Arsenic _____ 35. Ca _____________
16. Mercury _____ 36. Bi _____________
17. Copper _____ 37. Sr _____________
18. Cobalt _____ 38. Si _____________
19. Beryllium _____ 39. Ni _____________
20. Selenium _____ 40. Li _____________
CHM 2045C Required Element List:
http://www.fccj.us/chm2045/Elements/45ElementTable.htm
CHM 2045C Online Element Spelling
Homework:
http://www.fscj.me/chm2045/elementquiz/elementquiz.html
CHM
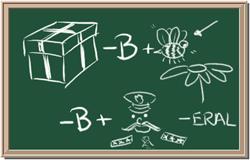
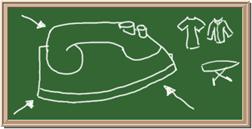
2045C Module One: Element Identification Homework 01 points
Identify each of the following elements chalk board representation:

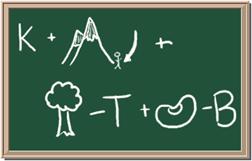
1. Element: _______________Symbol:_____ 2.Element: _______________Symbol:_____
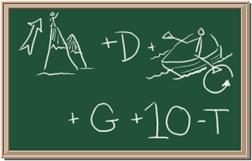
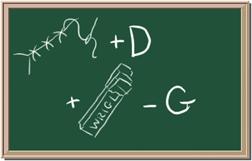
3. Element: _______________Symbol:_____ 4.Element: _______________Symbol:_____

5. Element: _______________Symbol:_____ 6.Element: _______________Symbol:_____
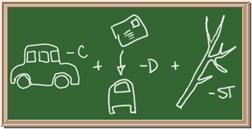
7. Element: _______________Symbol:_____ 8.Element: _______________Symbol:_____
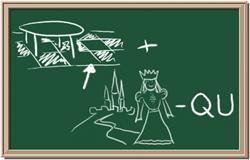
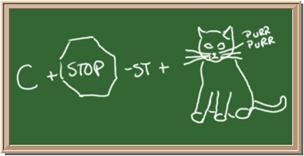
9 Element: _______________Symbol:_____ 10.Element: _______________Symbol:_____
Answers Web Site: http://www.chem4kids.com/files/elem_intro.html
CHM 2045C Module One Homework
Packet
M-1 Part C: Element Classification 05 points
Using
the periodic chart provided, in the blanks below write the classification of
the elements listed:
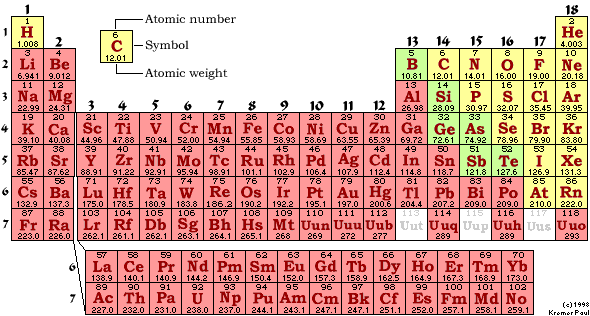
The
classifications of the elements are:
Metals;
Semimetals
(Metalloids);
Nonmetals-Regular;
Nonmetals-Nobel
Gases;
Nonmetals-Class
by Itself
Use
the above classifications to classify each of the following elements.
You
must split the nonmetals into three subclasses:
1. 12C6 ______________
2. 39K19 ______________
3. 11B5 ______________
4. 1H1 _________________
5. 4He2 _________________
6. 56Fe26 _________________
7. 238U92 _________________
8. 73Ge32
_________________
9. 9Be4 _________________
10. 86Rn222 _________________
CHM 2045C Module One Homework Packet
Part
C1: Compounds and Chemical Formulas 01 points
1.
State the Law of
Definite Composition:
2.
Define Molecule:
3.
The chemical
formula for vitamin B3 is: C6H6N2O
In One Molecule How Many:
_____ carbon
atoms
_____ oxygen
atoms
_____ total
atoms
4.
Write the
chemical formula for Vitamin B6 which has eight Carbon atoms, 11
Hydrogen atoms, one Nitrogen atom, and three Oxygen atoms:
How many
total atoms are there in one Vitamin B6 molecule?
5.
Citric Acid (in
citrus fruit) has the following chemical formula:
C3H4OH(COOH)3
In one
molecule of Citric Acid, How many:
______Carbon
atoms
______Oxygen
Atoms
______
Hydrogen Atoms
______ total atoms
Bonus: How many atoms are there in the following
complex ion compound:
[Cr(N2H4CO)6]4[Cr(CN)6]3 ________________total
atoms
CHM 2045C Module One Homework Packet
Part
F: Phase Diagrams 03 points
Identify the
points labeled on the
Phase Diagram of water:
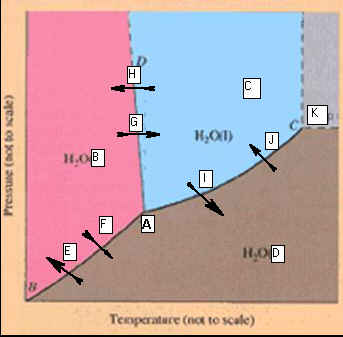
_____________________A.
_____________________B. _____________________H
_____________________C. _____________________I
_____________________D. _____________________J
_____________________E. _____________________K
_____________________F
_____________________G
CHM1025C Module One Homework Packet
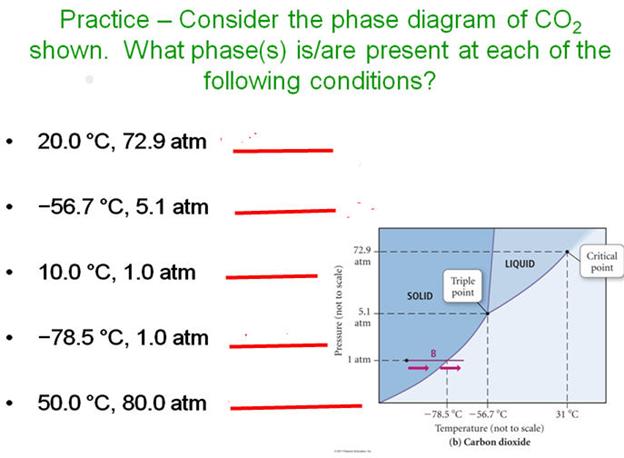
Phase Diagram for Carbon Dioxide.
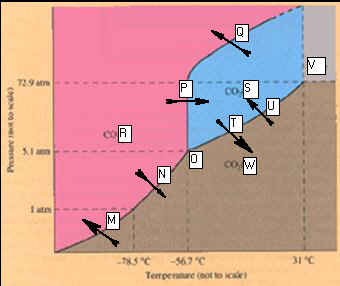
_____________________M.
____________________T
_____________________N. _____________________U
_____________________O. _____________________V
_____________________P. _____________________W
_____________________Q.
_____________________R
_____________________S
Corwin’s Phase
Diagram:
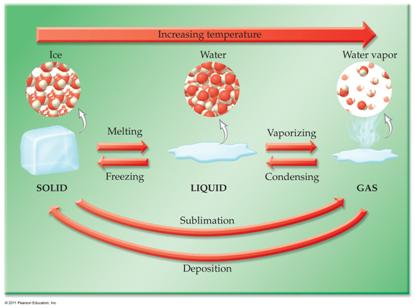
CHM1025C Module One Homework Packet
Part
P1: Phase Diagram Applications
1 point
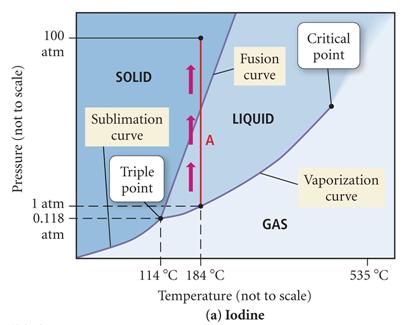
A demonstration of heating
iodine in a beaker has purple vapors..can you explain
using the phase diagram above?
Watch Video:
Real Video Movie
(Requires Real Video Plug In):