CHM 2045C Module 4 Homework Packet Name:_________________
Module Four Part I: Language of Chemistry/Chemical Bonds
A. _____(03) Bond Recognition/Compound Classification-Sections 2.8 Answers
B. _____(00*) Dot Structures of Molecules-Section
8.7 Answers
C. _____(03) Binary Molecular(Covalent)
Compounds-Section 2.8 Answers
D _____(03) Binary Ionic Compounds-Section 2.5-2.6 Answers
E. _____(02) Polyatomic Ions-Section
2.5-2.6 Answers e
F. _____(03) Ternary Ionic Compounds-Section
2.5-2.6 Answers f
G. _____(03) Binary Acids/ Ternary
Oxyacids-lecture, 4.5 Answers g
H. _____(06) Inorganic Compounds
Section 2.8 p97-98 Answers h
_______(23) Total = ______%
M-4 Required
Homework:
_____ (23) M-4 Pretest Hardcopy Homework Packet
______(30) M-4 Multiple Choice (MC) Homework/Exam (Blackboard
Online)
______(10) Polyatomic Ions Flash Card or Progressive
Polyatomic ion online Homework
______(03)
Jespersen Polyion Test
______(10) Polyatomic
Ions Progressive Test (Best Score of three attempts)
Required
List
_____
(20*) Hard Copy Dot Structure Homework/Lab:
Reference: Drag and Drop Interactive
Online: B: Dot Structure of
Molecules
______(60) Online Names/Formulas
Homework (Submit separate Goldenrod form-(Next Page)
Online Nomenclature Homework/Lab
C: Binary
Molecular Cpd Hmwk
C1. Binary
Molecular Formulas Hmwk
D: Binary
Ionic Cpd Hmwk
D1. Binary Ionic Formulas
E: Polyatomic
Ion Names Hmwk
E1: Polyatomic
Ion Formulas Hmwk
F: Ternary
Ionic Cpd Hmwk
F1. Ternary Ionic Formulas
G: Binary/Ternary
Acid Hmwk
G1. Acid Formulas
H: Inorganic
Cpd Hmwk
H1. Inorganic
Cpd Formulas Hmw
CHM 2045C Grading Name: ______________
Sample Dot
Structure/Nomenclature Lab Report
-------------------------------------------------------------------------------------------------
C: Binary Molecular Cpd Hmwk
______completed _____out
of 20 _______(05)
______not completed (______must complete by Exam2 _____did not complete)
C1.
Binary Molecular Formulas Hmwk
______completed _____out
of 20 _______(05)
______not
completed (______must complete by Exam2 _____did not complete)
D: Binary Ionic Cpd Hmwk
______completed _____out
of 20 _______(05)
______not completed
(______must complete by Exam2 _____ did not complete)
D1. Binary Ionic Formulas
______completed _____out
of 20 _______(05)
______not completed (______must complete by Exam2
_____
did not complete)
E: Polyatomic Ion Names Hmwk
______completed _____out of 20 _______(05)
______not completed (______must complete by Exam2
_____
did not complete)
E1: Polyatomic Ion Formulas Hmwk
______completed _____out
of 20 _______(05)
______not completed
(______must complete by Exam2 _____ did not complete)
F:
Ternary Ionic Cpd Hmwk
______completed
_____out of 20 _______(05)
______not completed (______must complete by Exam2
_____
did not complete)
F1. Ternary Ionic Formulas
______completed
_____out of 20 _______(05)
______not completed (______must complete by Exam2
_____
did not complete)
G:
Binary/Ternary Acid Hmwk
______completed _____out of 20 _______(05)
______not completed (______must complete by Exam2 _____ did not
complete)
G1.
Acid Formulas
______completed _____out
of 20 _______(05)
______not completed (______must complete by Exam2
_____
did not complete)
H: Inorganic Compound Hmwk
______completed
_____out of 40 _______(05)
______not completed (______must complete by Exam2
_____
did not complete)
H1. Inorganic Compound Formulas Hmwk
______completed _____out
of 40 _______(05)
______not completed (______must complete by Exam2
_____
did not complete)
_______(60) Total
Points
________(10)
Polyatomic Flash cards and/or Online Progressive Polyatomic Ion Practice
http://www.northcampus.net/Nomenclature/PolyatomicIonFormula/ProgressivePolyatomicIonFormula.html
________(03) Table 2.4Jespersen’s Polyatomic Ion List (Section
2.5-2.6)
________(10) Best Grade Progressive Polyatomic Ion Test
1st Attempt at Progressive polyatomic ion Test:
_____ out of (50)
2nd Attempt at Progressive polyatomic ion Test: _____ out of (55)
3rd Attempt at Progressive polyatomic ion Test: _____ out of (60)
CHM
2045C Module 4 Homework Packet
Module
Four: Part A Sample
Bond Recognition 3 points
Using
a periodic chart, predict the bond that would form between the two elements:
Sample test: Answers on Grading Outline
1. Fe-Al ________________
2. P-S ________________
3. C-O ________________
4. B-Cl ________________
5. K-I ________________
For the following element pairs use the electronegativity table below to determine if the bond is ionic or covalent.

6. Na-P ________________
7. Ca-Br ________________
8. Ge-O ________________
9. P-H ________________
10. Be-Cl ________________
Text
Reference Sections 2.8 + Study Guide:
http://www.fccj.us/chm1025/AssignmentOutline/M4PartA.htm
Module
Four: Part B Dot Structures of Molecules 0 points
Using
a periodic chart draw the electron dot structures of the following molecules:
(Choose One for each
question or the one circled on the paper)
1. NH3 CH4 H2O2 H2O 2.
H2SO4 H3PO4 HClO4 HClO3
Submit these dot
structures as a separate homework
3. HNO3 H2CO3 HNO2 4.
CO2 HCN SO3 SO2
Submit these dot
structures as a separate homework
5. HC2H3O2 H2C2O4 HCHO2
6.
C2H4 C2H2 C3H8 C2H6
carbon to carbon by single
covalent bond bond
carbons to carbon
Submit these dot
structures as a separate homework
7. CH3CH2OH CH3COCH3 CH2O (HCHO)
(carbon to
carbons by single covalent bonds-oxygen attach to carbon)
Submit these dot
structures as a separate homework
8. CH3OCH3 CHONH2 CH3CH2CH2OH CH3CHOHCH3
oxygen
separates the carbons O & N
both bond to C (all three
carbons single bonded and –OH attached to carbon)
Submit these dot
structures as a separate homework
9. CH2NH2COOH CH3CHNH2COOH
carbon to carbons by
single covalent bonds (-NH2 amino on#2 carbon in both above)
Submit these dot
structures as a separate homework
10. CH3COOCH2CH3 HCOOCH3
(-CH2CH3 also
hooks to oxygen in#10, as well as - CH3 )
Submit these dot structures as a separate homework 20
points
See handout for directions
Drag and Drop Interactive Web Site:
http://www.lsua.us/chem1001/dragdrop/menu.html
CHM 2045C Module 4 Homework Packet
Module Four:
Part C Binary Molecular Compounds 3 points
Using
a periodic chart write the names or formulas of the following compounds
depending on whether the formula or name is given:
Sample test: Answers on Grading Outline
1. CO
____________________
2. SO3 _____________________
3. N2O5 _____________________
4. N2O7 _____________________
5. N2O _____________________
6. Phosphorus pentachloride _________
7. Boron trifluoride _________
8. Carbon dioxide _________
9. Sulfur Trioxide _________
10. Carbon Tetrachloride _________
Textbook Reference: McMurry Section 2.12/Jespersen
Section 2.8
Online
Homework (see grading form):
C:
Binary Molecular (Covalent) Homework: http://www.northcampus.net/Nomenclature/Molecules/45BinaryCovalent.html
C1.
Binary Molecular (Covalent) Formulas: http://www.northcampus.net/Nomenclature/MoleculeFormula/45BinaryMolecularFormula.html
Submit
grades on separate grading Sheet when taking M-4 Exam
Online
Study Guide:
http://www.fccj.us/chm1025/AssignmentOutline/M4PartC.htm
Module 4 Homework Packet
Module
Four: Part D Binary Ionic
Compounds 3 points
Using
a periodic chart, write the names or the balanced formulas for the following
compounds depending on whether the formula or the name is given:
Sample test: Answers on Grading Outline
1. Copper II phosphide _________ (Cupric phosphide)
2. Iron III Oxide (rust) _________ (Ferric Oxide)
3. Lead IV sulfide _________ (Plumbic sulfide)
4. Sodium chloride _________
5. Tin II fluoride (in toothpaste) _________ (Stannous Fluoride)
6. MgCl2 ________________________
7. NiF2 ________________________
8. K3N ________________________
9. Al2O3 ________________________
10.
CuBr ________________________
Reference:
McMurry Section 2.12/Jespersen Section 2.5-2.6
Online
Homework (see grading form):
D:
Binary Ionic Compound Homework: http://www.northcampus.net/Nomenclature/BinaryIonicName/45BinaryIonic.html
D1.
Binary Ionic Formulas:
http://www.northcampus.net/Nomenclature/BinaryIonicFormula/45BinaryIonicFormula.html
Submit
grades on separate grading Sheet when taking M-4 Exam
Online Study Guide:
http://www.fccj.us/chm1025/AssignmentOutline/M4PartD.htm
Module 4 Homework Packet
Module
Four: Part E Polyatomic Ions 2 points
Using
a periodic chart write the names or formulas of the following polyatomic ions
depending on whether the formula or name is given:
1. CO32- _____________________
2. SO32- _____________________
3. PO33- _____________________
4. ClO31- _____________________
5. NO31- _____________________
6. Hydroxide ________
7. Ammonium ________
8. Hypochlorite ________
9. Nitrite ________
10. Phosphate ________
Textbook Reference:
McMurry
Section 2.12/Jespersen Section 2.5-2.6
Online
Homework (see grading form):
E:
Polyatomic Ion Names Homework: http://www.northcampus.net/Nomenclature/PolyatomicIon/25PolyatomicIon.html
E1.
Polyatomic Ion Formulas: http://www.northcampus.net/Nomenclature/PolyatomicIonFormula/45PolyatomicIonFormula.html
Submit
grades on separate grading Sheet when taking M-4 Exam
Jespersen’s Table 2.4 Section 2.5 Polyion Test #1
Flash Cards 10 points (Show on M-4 Exam for credit) or online at the following:
http://www.northcampus.net/Nomenclature/PolyatomicIonFormula/ProgressivePolyatomicIonFormula.html
Progressive Polyatomic Ion Test 10 Points Total Test#2; #3; Possible #4
See Required List on the next pages
Module 4 Homework Packet
Jespersen Table of Common Polyatomic Ions
Progressive Polyatomic Ion Test #0
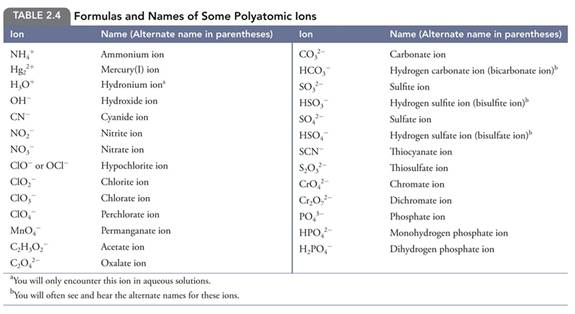
McMurry
Table of Polyatomic Ions
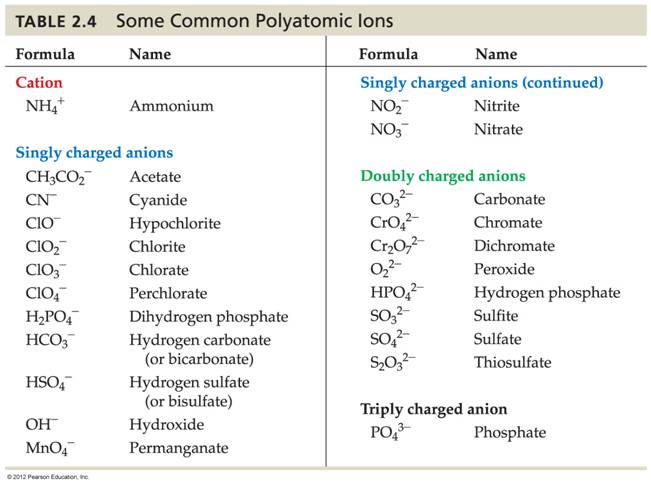
Required List of Polyatomic Ions – CHM 2045C
|
Borate BO33− Carbonate
CO32− Carbonite
CO22− Silicate SiO32− Stannate
SnO32− Stannite
SnO22− Plumbate
PbO32− Plumbite
PbO22− Nitrate NO3− Nitrite NO2− Phosphate
PO43− Phosphite
PO33− Arsenate AsO43− Arsenite AsO33− Antimonate SbO43− Antimonite SbO33− Bicarbonate HCO3− Hydrogen carbonate
HCO3− Hydrogen arsenate
HAsO42− Hydrogen phosphate HPO42− Hydrogen phosphite
HPO32− Hydrogen sulfate HSO4− Hydrogen sulfite HSO3− Dihydrogen Arsenate
H2AsO4− Dinhydrogen Phophate
H2PO4− Dihydrogen Phosphite H2PO3− Bromate BrO3− Bromite BrO2− Chlorate
ClO3− Chlorite
ClO2− Iodate IO3− Iodite IO2− Ammonium NH4+ Hydronium H3O+ Mercury
(I) Hg22+ |
Selenate
SeO42− Selenite
SeO32− Sulfate
SO42− Sulfite
SO32− Tellurate
TeO42− Tellurite
TeO32 Hypobromite
BrO− Hypochlorite
ClO− Hypoiodite
IO− Hypophosphite
PO23− Hyposulfite
SO22− Periodate IO4− Perbromate
BrO4− Perchlorate
ClO4− Azide N3− Carbide C22− Peroxide
O22− Acetate
C2H3O2− Cyanate
OCN− Oxalate
C2O42− Thiocyanate
SCN− Cyanide
CN− Hydroxide
OH− Chromate
CrO42− Dichromate Cr2O72− Ferrate
FeO42−
Molybdate
MoO42− Permanganate MnO4− Tungstate
WO42− Vanadate
VO43− |
|
Module
Four: Part F Ternary Ionic
Compounds 3 points
Using
a periodic chart write the names or formulas of the following compounds
depending on whether the formula or name is given:
1. Na2CO3 _____________________
2. K2SO4 _____________________
3. (NH4)3PO4 _____________________
4. Ca(ClO3)2 _____________________
5. CuNO3 _____________________
6. Aluminum Hydroxide ____________
7. Ammonium carbonate ____________
8. Sodium Hypochlorite ____________
9. Magnesium Nitrate ____________
10. Iron III sulfite _____________
Text Reference: McMurry Section 2.12/Jespersen
Section 2.5-2.6
Online
Homework (see grading form):
F:
Ternary Ionic Compound Names Homework: http://www.northcampus.net/Nomenclature/TernarySalts/45ternaryIonic.html
F1.
Ternary Ionic Compound Formulas: http://www.northcampus.net/Nomenclature/TernarySaltFormula/45ternaryionicformula.html
Submit
grades on separate grading Sheet when taking M-4 Exam
Module 4 Homework Packet
Module
Four: Part G Binary/Ternary Acids 3 points
Using
a periodic chart write the names or formulas of the following compounds
depending on whether the formula or name is given:
1. HCl
_____________________
2. H2SO4 ____________________
3. HNO3 _____________________
4.
HNO2 ___________________
5. H2CO3 ___________________
6. Hypochlorous acid _________
7. Phosphoric acid _________
8. Sulfurous acid _________
9. Perchloric acid _________
10. Hydrofluoric acid ________
McMurry Text Section 4.5/Jespersen
Text Section 4.5
Online
Homework (see grading form):
G:
Binary/Ternary Acid Names Homework: http://www.northcampus.net/Nomenclature/Acids/45acids.html
G1.
Binary/Ternary Acid Formulas:
http://www.northcampus.net/Nomenclature/AcidFormulas/45AcidFormulas.html
Submit
grades on separate grading Sheet when taking M-4 Exam
Online Study Guide:
http://www.fccj.us/chm1025/AssignmentOutline/M4PartG.htm
Module
Four: Part H Inorganic Compounds 6 points
Using
a periodic chart write the names or formulas of the following polyatomic ions
depending on whether the formula or name is given:
1. H2CO3 _____________________
2. MgSO4 _____________________
3. Ca3(PO3)2 ____________________
4. HClO3 _____________________
5. SO3 _____________________
6. Fe2O3 _____________________
7. NaHCO3 ______________________
8. CuNO3 ______________________
9. K2Cr2O7 ______________________
10.
P4O10
______________________
11.
H3PO4
_______________________
12. CO2 ________________________
13. NaClO
________________________
14. Pb(C2H3O2)4 ________________________
15. Ni(NO3)3 _________________________
16. Aluminum Hydroxide ____________
17. Ammonium chloride _____________
18. Sodium Hypochlorite _____________
19. Nitrogen dioxide _____________
More
on next page
20. Calcium Phosphate ____________
21. Sulfuric acid ____________
22.
Sodium Hydroxide ____________
23.
Ammonia
____________
24.
Dihydrogen Monoxide ____________
25.
Hypochlorous Acid ____________
26.
Silver Nitrate
____________
27.
Potassium Cyanide ____________
28.
Acetic Acid
____________
29.
Barium Sulfate
____________
30.
Tin II Fluoride
____________
Text: McMurry Sections 2.12 and 4.5/Jespersen
Section 2.8
Online
Homework (see grading form):
H:
Inorganic Compound Names Homework: http://www.northcampus.net/Nomenclature/Inorganic/45inorganic.html
H1.
Inorganic Compound Formulas:
http://www.northcampus.net/Nomenclature/InorganicFormula/45inorganicFormula.html
Submit
grades on separate grading Sheet when taking M-4 Exam
Online Study Guide:
http://www.fccj.us/chm1025/AssignmentOutline/M4PartH.htm