CHM 2045C Name: _________________
Module Five II Homework Packet
Module Five
Part II: Chemical Equations &
Stoichiometry (Chapter 3)
A._____(05)
Molecular Mass
Calculation-Section 3.3 Answers a
B ._____(05) Mole Calculations I-Sections 3.3 Answers
B1.____(05) Mole Calculation II-Section 3.3: Answers
bcd
C._____(05) Percentage Composition
Calculation-Section 3.10 Answers bcd
D._____(05) Empirical Formula Calc. from Lab Data-Section 3.11 Answers bcd
D1.____(05) Empirical Formula
Calc. from % Comp-Section
3.11 Answers
________(30) Total M-5ii Homework Packet
M-5ii
Required Homework:
______(30) M-5ii
Pretest Hardcopy Homework Packet
______(25) M-5ii Multiple Choice (MC)
Practice (Blackboard Online)
Module Five-Part A: Molecular Mass Calculation 5 points
The atomic mass of any substance expressed in grams is
the molar mass (MM) of that substance.
•
The
atomic mass of carbon is 12.01 amu per atom.
•
Therefore,
the molar mass of carbon is 12.01 g/mol .
•
Since
nitrogen occurs naturally as a diatomic, N2, the molar mass of
nitrogen gas is two times 14.01
g or 28.02 g/mol.
Calculating
Molar Mass
•
The
molar mass of a substance is the sum of the molar masses of each element.
•
What
is the molar mass of copper(II) nitrite, Cu(NO2)2?
•
The
sum of the atomic masses is as follows:
63.55 + 2(14.01 + 16.00 +
16.00) =
63.55 + 2(46.01) = 155.57 amu per molecule
•
The
molar mass for Cu(NO2)2 is
155.57 g/mol.
Homework #1 (M-5 A):
Using a periodic
chart calculate the molar mass of the following:
1. Calculate the molecular
mass of Acetic Acid, HC2H3O2.
2. Calculate the
formula unit mass of Ammonium Chromate,
(NH4)2CrO4 .
3. Calculate the
molecular mass of glucose,
C6H12O6.
Reference: Section 3.3 page 79
Additional Problems: #3.4 Page83
End of Chapter Page 106 Questions 3.44-3.54
Interactive Online Chem-i-Calc(Molar Mass & %
Composition):
http://people.emich.edu/bramsay1/ccc-release/chem.html
Module
Five II Homework Packet
Part B: Mole Calculation 1 5 points
Your text in Section 3.1 does not address too well M-5B and M-5B1
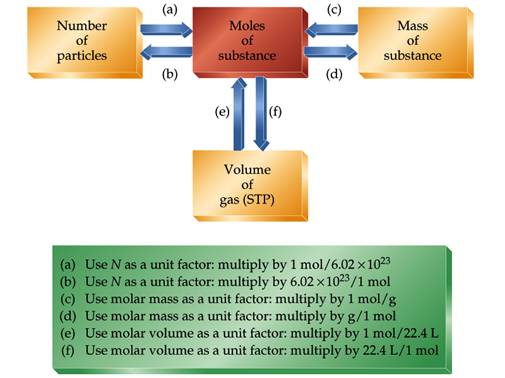
This is the only time in the course you will need to use Avogadro's number to relate actual particles to masses and volumes. In the lab we move from masses to mole and never focus on the number of particles.
What
is a mole?
•
The mole (mol) is a unit of measure
for an amount of a chemical substance (chemist dozen).
•
A mole is Avogadro’s number of particles,
which is 6.02 x 1023 particles.
1 mol = Avogadro’s number = 6.02 x 1023 units
(atoms, Molecules, ions, e1-)
We can use the mole relationship to convert between the
number of particles and the mass of a substance
Example
B1: How many sodium atoms are in 0.240 mol Na?
Question :
_____?______Na Atoms = 0.240 mol Na
1.
We want atoms of Na.
2.
We have 0.240 mol Na.
3.
1 mole Na = 6.02 x 1023 atoms
Na.
Solution:
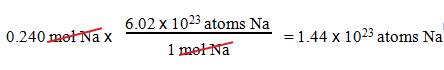
Homework #2 (M-5B):
B1: How many Carbon atoms are contained in 0.01667 moles
of Carbon ( 1 carat diamond)?
Example
B2: How many moles of aluminum are in 3.42 x 1021 atoms Al?
Question :
_____?______Mole Al = 3.42 x 1021 atoms Al
1.
We want moles Al.
2.
We have 3.42 x 1021 atoms Al.
3.
1 mol Al = 6.02 x 1023 atoms
Al.
Solution:
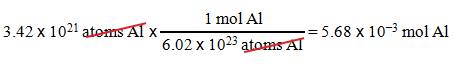
Homework #3 (M-5B):
B2: How many moles of Carbon are contained in 3.016 x 1022
atoms of Carbon (3 carat diamond)?
Part B1: Mole calculation II 5 points
We will be using the unit analysis
method again.
Recall the following steps:
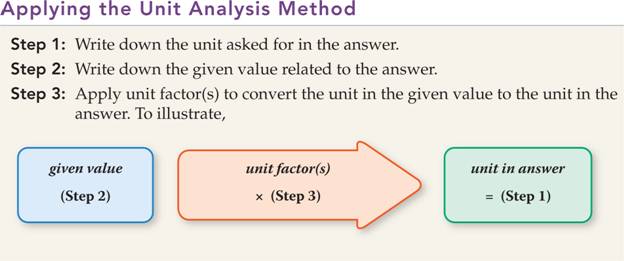
Mole Calculations II
Now we will use the molar mass of a
compound to convert between grams of a substance and moles or particles of a
substance.
6.02 x 1023
particles = 1 mol = molar mass
If we want to convert particles to
mass, we must first convert particles to moles, and then we can convert moles
to mass.
Example B3: What is the mass of 1.33
moles of titanium, Ti?
1. We
want grams.
2. We
have 1.33 moles of titanium.
3. Use
the molar mass of Ti: 1 mol Ti = 47.88 g Ti.
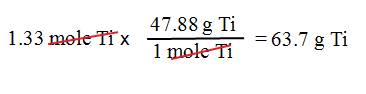
Atoms–Mass Calculation
Example B4: What is the mass of 2.55 x 1023
atoms of lead?
1. We
want grams.
2. We
have atoms of lead.
3. Use
Avogadro’s number and the molar mass of Pb.
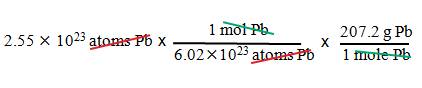
= 87.9 g Pb
Mass–Molecule Calculation
Example B5: How many F2
molecules are present in 2.25 g of fluorine gas?
1. We
want molecules F2.
2. We
have grams F2.
3. Use
Avogadro’s number and the molar mass of F2.
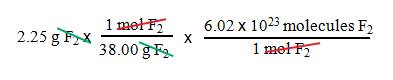
= 3.56 x 1022
molecules F2
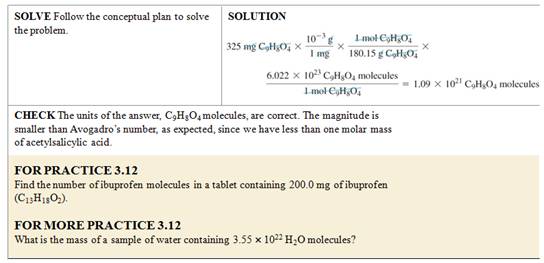
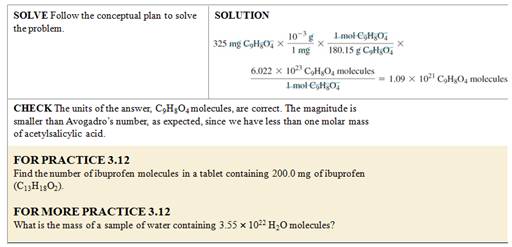
Mass of an Atom or Molecule
•
What is the mass of a single
molecule of sulfur dioxide? The molar
mass of SO2 is 64.07 g/mol.
•
We want mass/molecule SO2, we have the molar mass of sulfur dioxide.
•
Use Avogadro’s number and the
molar mass of SO2 as follows:
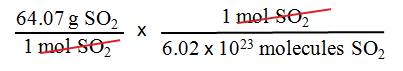
= 1.06 x 10–22 g/molecule
Homework #4 (M-5B1):
Calculate the mass in grams of 1.16 x 1022 molecules of nitrogen gas, N2
Homework #5 (M-5B1):
Calculate the number of entities in 0.641 g of oxygen gas, O2
Text reference: page 80
Conceptual Problem 3.5 page 83
For part B additional end of chapter problems may be found on page 106 questions 3.55-3.59
Part C:
Percent Composition 5 points
The percent composition of a compound lists the
mass percent of each element.
For example,
the percent composition of water, H2O, is 11% hydrogen and 89%
oxygen.
All water contains 11% hydrogen and 89% oxygen by mass.
Calculating Percent Composition of
water
There are a few steps to calculating the percent
composition of a compound. Let’s
practice
using H2O.
- Assume you have 1 mol
of the compound.
- One mole of H2O
contains 2 mol of hydrogen and 1 mol of oxygen. Therefore,
2(1.01
g H) + 1(16.00 g O) = molar mass H2O
2.02
g H + 16.00 g O = 18.02 g H2O
- Next, find the percent
composition of water by comparing the masses of hydrogen and oxygen in
water to the molar mass of water.
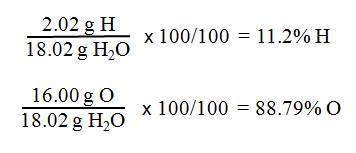
- Remember: g H/100 g H2O is % H
and g O/100 g H2O is % O
Example #2: Percent
Composition Problem
TNT (trinitrotoluene) is a white
crystalline substance that explodes at 240 °C. Calculate
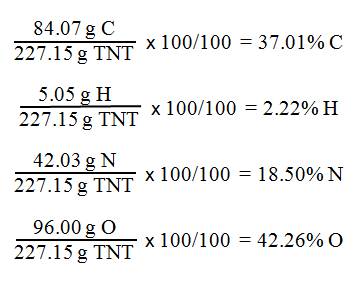
the percent composition of TNT, C7H5(NO2)3.
First
calculate the molar mass of TNT
7(12.01 g C)
+
5(1.01 g H)
+
3 (14.01 g N + 32.00 g O) = g C7H5(NO2)3
84.07 g C
+ 5.05 g H
+
42.03 g N
+
96.00 g O
227.15 g C7H5(NO2)3
Second Calculate the % Composition
Module
5 Part C is covered in Section 3.10-3.11 of the textbook,
pages _____, calculating the Percent Composition of a Compound. You will
calculate the Percent Composition of one compound on M-5 Exam.
Homework Problem #6 (M-5C):
Calculate the percent composition of each
element in the following compound:
Acetic
Acid HC2H3O2
Text Reference: Section 3.10 Pages 94-97
see worked example 3.16 pages 96-97 try problem 3.24 page 97
There is an interactive web site that will allow you to check your
work for Parts A & C. The author of ChemiCalc
(which I posses two site licenses if you want a copy),
Dr. Bert Ramsay of
http://people.emich.edu/bramsay1/ccc-release/chem.html
For additional examples at the end of the chapter do the Percent
Composition on page 108-109 Questions #3.98-3.99
Part D:
Empirical Formula Calculation
5 points
What is the Empirical Formula?
The empirical formula of a
compound is the simplest whole number ratio of ions in a formula unit or atoms
of each element in a molecule.
The molecular formula of benzene is C6H6.
- The
empirical formula of benzene is CH.
The molecular formula of octane is C8H18.
- The
empirical formula of octane is C4H9
Calculating Empirical
Formulas from % Composition
We can also use percent composition
data to calculate empirical formulas.
•
Assume that you have 100 grams of
sample.
Acetylene is 92.2% carbon and 7.83%
hydrogen. What is the empirical formula?
First:
If we assume 100 grams of sample,
we have 92.2 g carbon
and 7.83 g hydrogen
Second: Calculate the moles of each
element.
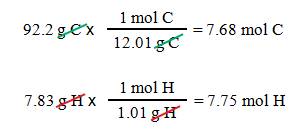
Third: The ratio of elements in
acetylene is C7.68H7.75.
Fourth:
Divide by the smallest number to get the following formula:
![]()

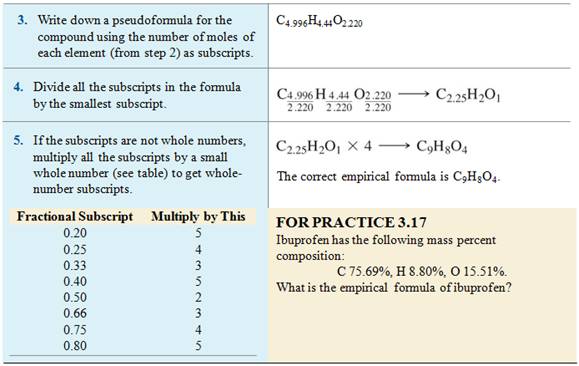
Homework #7 (M-5D) Empirical
Formula via % Composition:
Given the following
percent compositions, determine the empirical formula of the following
compound: 54.5% carbon, 9.15% hydrogen, and 36.3% oxygen
Text Reference Section 3.10 see worked example 3.15 page 96
Additional problems: 3.22-3.23 p97; end chapter Q# 3.100-3.103 p109
Calculating Empirical
Formulas from Lab Data
We can calculate the empirical formula
of a compound from its composition data upon analysis in the lab.
We can determine the mole ratio of each
element from the mass to determine the empirical formula of radium oxide, Ra?O?.
Example #1
A
1.640 g sample of radium metal was heated to produce 1.755 g of radium
oxide. What is the empirical formula?
– We have 1.640 g Ra and 1.755 – 1.640
= 0.115 g O
– The molar mass of radium is 226.03
g/mol, and the molar mass of oxygen is 16.00 g/mol.
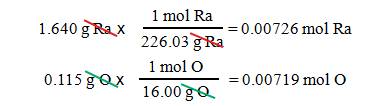
- We
get Ra0.00726O0.00719.
- Simplify
the mole ratio by dividing by the smallest number,0.00719
into both 0.00726 and .00719.
- We
get Ra1.01O1.00
- Although
we use at three significant figures throughout the calculation, empirical
formulas are rounded to one significant figure resulting in whole number ratios
- Answer: RaO is the empirical
formula
We may do a similar
calculation in the lab: Determining the Empirical Formula
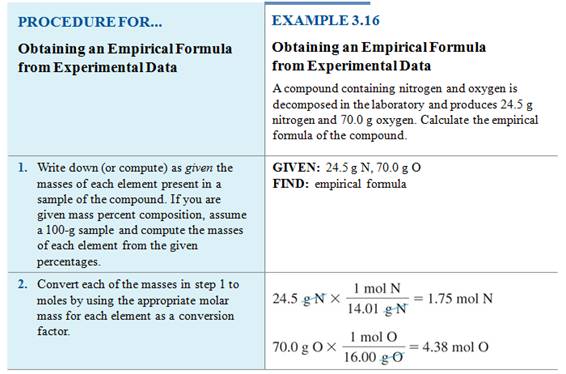
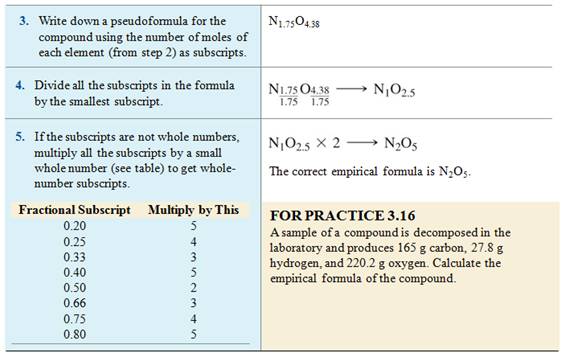
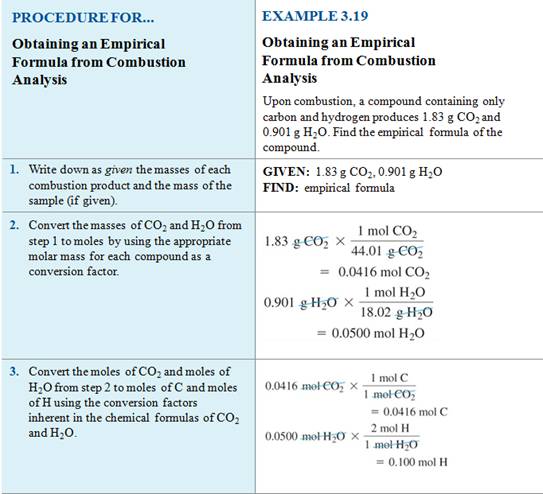
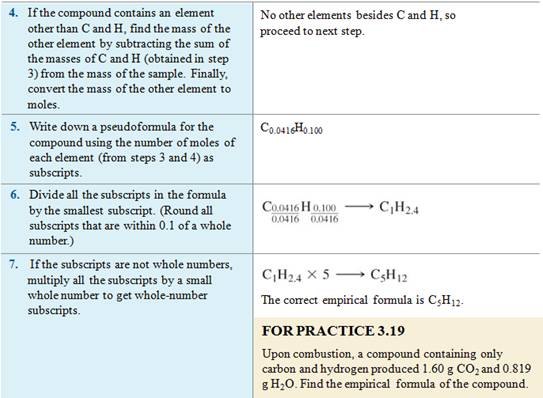
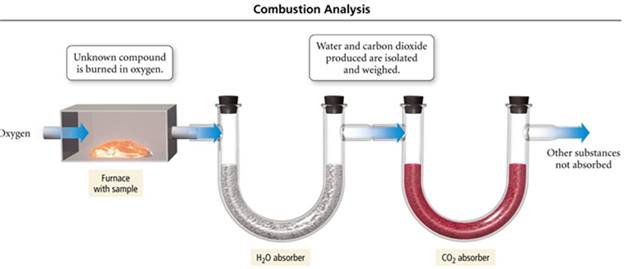
Homework #8 (M-5D1) Empirical Formula
via Lab Data:
In an experiment 2.410 gram of copper
oxide produced 1.925 g of copper metal after heating with oxygen gas. What is
the empirical formula of copper oxide?
Text Reference: Section 3.11
pages 97-99
See additional worked example
3.17 page 99;also work 3.25, 3.26, 3.27 page 99
There are empirical formula problems at the end of the chapter
to practice your empirical formula calculation of a compound: page 109 problems
#3.104-3.107.