CHM 2045C Name: _________________
Module Six Homework Packet
Module Six: The Gaseous State (Chapter 9) Assignment Outline
A._____(05) Kinetic Molecular
Theory-Section 9.6 Answer ppt9
B._____(05) Discussion Real vs Ideal Gas Equation-Sect 9.8 Answer bc
C._____(05) Standard
Conditions/Molar Volume-Sect 9.1-9.2 Answer bc
D._____(05) Gas Laws/Vocabulary-Sections
9.2-9.3, 9.5, 9.7 Answers
E._____(10) Gas Law Problems-Sections
9.2-9.3, 9.5, 9.7 Answers
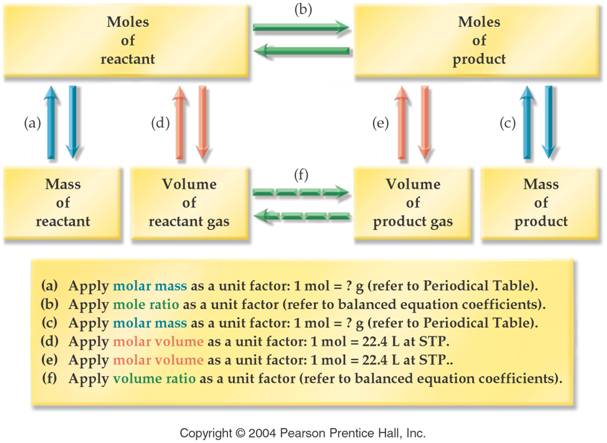
F. _____(05) Volume-Volume Stoichiometry Problem-Section 9.4 Answers fg
G._____(05) Mass-Volume Stoichiometry Problem-Section 9.4 Answers fg
H._____(05) Gas
Densities/Molecular Mass Determination-Sect 9.4 Answers
______(50) Total =
______%
Module Six- Part A: Kinetic Molecular Theory 5 points
State the 5
assumptions of the Kinetic Molecular theory as stated in the book (Section 9.6) :
1
2
3
4
5.
For shorter answers reference section 10.10 page
299-300 and write only the bold black sentences
Module
Six Homework Packet-Page 2
Module Six Part B: Discussion Question 5 points
In the Real Gas Equation:
(P + an2/V2) (V - nb) = n RT a pressure correction factor was added. Why?
(What assumptions of the kinetic theory breakdown under extreme conditions of
temperature and pressure?)
Also a volume correction factor was subtracted. Why? (What
assumptions of the KMT breakdown under extreme conditions?)
Module Six-Part C
Standard Conditions/Molar Volume
5 points
State standard conditions (STP) in three units of pressure
(the last is your choice) and oC and K temperatures:
_____mm Hg or ______torre= ______atm = _____ ______(you write the unit too)
_____ oC =
______K
Are the values for the Molar Gas Volume Constant:
1 mole CO2 =________L
CO2@STP 1 mole
H2 =________L H2@STP
1 mole N2 =________L
N2@STP 1 mole
O2 =________L O2@STP
Reference:
Sections
9.1-9.2
Module
Six Homework Packet Page 3
Module Six Part D Gas Laws 05
points
State:
Boyle’s Law (In words and formula)
Charles Law (in words and formula)
Dalton’s Law of Partial pressures (in words and formula)
Gay-Lussac’s Law (in words and formula)
Avogadro’s Law (in words and formula)
Combined Gas Law Equation (write only the equation)
Ideal Gas Equation (write only the equation)
Define Vapor Pressure
Graham’s Law of Diffusion(in words
and formula)
Module
Six Homework Packet – Page 4
Module Six Part E Gas Law Problems 10 points
Boyle’s Law (See Section 10.4 and Example 10.3)
(Additional Problems p307-8 #15-20)
1. A sample of a gas has a volume of 100 mL when measured at 25 oC
and
760 mmHg. What volume will the gas occupy at 25 oC and 380 mmHg?
Charles Law(See Section 10.5 and Example 10.4) (Additional Problems p308
#21-26)
2. The volume of a gas is 100.0 mL
at 27 oC. At what temperature in degrees Celsius would
the volume of the gas be 200.0 mL, assuming the
pressure remains constant.
Gay-Lussac’s Law (See Section 10.6 and Example 10.5)
(Additional Problems p308 #27-32)
3. A sample of gas
occupies 100.0 L at 710.0 torre and 27 oC.
Calculate the pressure in torre if the
temperature is changed to 127 oC
while the volume remains constant.
Dalton’s Law of Partial Pressures (See Section
10.4 & Example 10.3) (Additional Problems p307-8 #15-20)
4. Calculate the dry volume in milliliters of 200 mL of hydrogen gas collected over water at 25 oC at 760 torre
pressure with the temperature remaining constant. (The partial pressure of water vapor at 25 oC is 23.8 torre.)
Avogadro’s Law
(See
Pages 230, 250, and 271)
5. A 1.5 mole sample
of a gas occupies 25.0 L at 758 torre and 27oC.
Calculate the Volume of the gas, if more molecules are injected into the vessel
increasing the moles to 2.5 moles, provided the pressure and the temperature do
not change.
Combined Gas Laws (See Section 10.7 and Example 10.6)
(Additional Problems p308 #33-42)
6. A100.0 mL sample of air is
collected at 25oC and 774 mmHg. What is the volume at STP?
Ideal Gas Equation (See Section 10.11) (Additional
Problems p308 #63-66)
7. Calculate the number of moles of nitrogen gas in a 5.00 L
cylinder at 27 oC and 4 atm pressure. R = 0.0821 L atm/ K
mole )
How much does this volume of gas weigh?
Module
Six Homework Packet – Page 5
Part F Volume-Volume Stoichiometry
5 points
In the Haber process, nitrogen
N2 and hydrogen H2 gases combine to give ammonia
gas NH3 as the only product. If 5.55 L of nitrogen gas
completely reacts, calculate the volume of ammonia that is produced. Assume all
volumes of gas are measured under constant conditions of 500 oC and 300 atm
pressure. How many liters of hydrogen are required to complete react the 5.55L
of nitrogen?
Part G Mass-Volume Stoichiometry 5 points
Potassium chlorate is used in the lab to make oxygen gas by
the following (unbalanced) reaction:
KClO3 à KCl + O2
How liters of oxygen may be made from reacting 1.226 grams of Potassium chlorate?
Module
Six Homework Packet – Page 6
Module Six Part H-1 Gas Density Problem 5 points
Calculate the gas density of octane
gas C8H18 vapor at STP?
Calculate the gas density of octane
gas C8H18 at 27 oC
and 750 torre?.
(Hint calculate the volume using
the ideal gas equation)
Module Six Part H-2 Molecular Mass Determination
Problem
Calculate the molecular weight of an unknown liquid that
when vaporized at 99 oC and 755 torre ,
gave 125.0 mL of vapor with a mass of 0.673
grams .
Module
Six Homework Packet – Page 7
Module Six Part I Effusion of Gasses Problem 5 points
A sample of Nitrogen gas escapes through a
tiny hole in 44.0 seconds. An
unknown gas escapes under the same conditions in 80.0 seconds. Calculate the molecular mass of the unknown.