CHM
2045C Module-4iii Homework Packet Name:
____________________
Please
complete the following homework sections before you attempt the exam. This is
homework. You grade it. This completed packet is due the day of the exam. No
credit for a section if the sample problem is shown and you leave any
additional problems which do not show the answer blank.)
Module Four Part III:
Chemical Bonding & Molecular Structure (Chapters 8-9)
B1 _____(07)
Lewis Dot/Stick Structures via Formal Charge Steric #2-#6 Section 8.7 Answers
L1. ____ (01) Bond
Angles/Bond Lengths Steric #5&6-Section 9.2 Answers
M._____ (00)
Molecular Orbitals Section 9.7-9.8
N1. ____ (01) Geometry
of Molecules-Steric #5&6 Section 9.1-9.2 Answers
O1. ____ (01) Polarity
of Molecules- Steric #5&6 Section 8.6,
9.3 Answers
P1. ____ (01) Hybrid
Orbital Recognition Steric #5&6-Sect 9.5-6 Answers
P2._____(00) Valence Bond Theory
Section 9.4
Q. _____ (01) Formal
Charge-Section 8.7 page 377-384 Answers
R. _____ (01) Resonance
Structures Section 8.8 Answers
S. _____ (01) Sigma/Pi Bonding
Section 9.6 Answers
______(14) Total
Module
Four III: Part B1 Dot Structures of Molecules Review 7 points
Using
a periodic chart draw the electron dot/stick structures of the following
molecules. Use the method of formal charges to show the best structure: (Steric
Numbers 2-6)
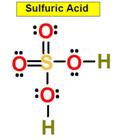
H2SO4 Also look at: H2SO3
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H3PO4 Also
look at: H3PO3
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HClO4 Also Look at: HClO3 HClO2 HClO
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
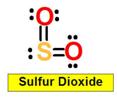
SO2
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
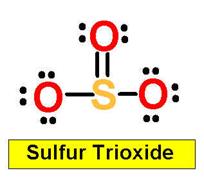
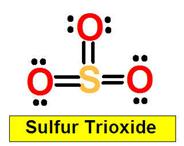
SO3
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SF6
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
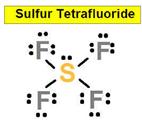
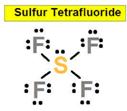
SF4
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SbCl5
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PCl5 Also look at PCl3
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AsF5
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SeF6
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HArF
(first Nobel Gas Molecule)
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Br31-
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
XeF4
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
XeO4
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BrCl3
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PCl61-
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BrF41+
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SO2Cl2
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
KrF2
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val Electrons |
- |
Bonding e1-
2 |
= |
Total |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
Ions/Compounds to consider which may be the exam question:
ClF5
AsF61-
SeF4 PF3Cl2 I31- BrF5
Reading
Reference: Sections 8.5 Octet Rule and 8.7 Formal Charge (page 374)
Reference
Octet Rule: B. Dot Structures of Covalent Compounds Section
2.10, 6.6, 7.1, 7.5, 7.6
Octet Rule Answers:
http://www.fccj.us/chm2045/SampleTest/45M4bAnswers.htm
Module Four: Part L1 Bond Angles 1 point
Steric Numbers #2--#6. What is the
bond Angle in the following structures:
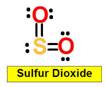
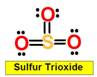
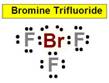

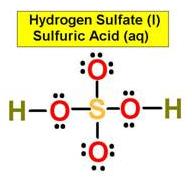
______1. Bond Angle between O=S=O in Sulfur dioxide
______2. Bond Angle between any O = S = O in Sulfur trioxide
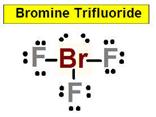
______3. Bond Angle between any F Br F in Bromine Trifluoride
______4. Bond Angle between any O = Xe = O in Xexon Tetroxide
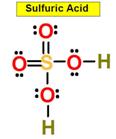
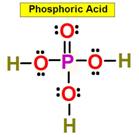
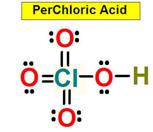
_____5. Bond Angle between either O = S
O in Sulfuric Acid
_____6. Bond Angle Between either O = P O in
Phosphoric Acid
_____7. Bond Angle Between
either O = Cl = O in Perchloric Acid



_____8. Bond Angle between any F Xe F in Xexon Tetrafluoride
____10. Bond Angle between any
equatorial F Br F in Bromine Pentafluoride
____11. Bond Angle between axial F Br and any equatorial F-B in Bromine
Pentafluoride
____12. Bond Angle between any equatorial F As F in Arsenic Pentafluoride
____13. Bond Angle between either axial F As and any equatorial F- As in
Arsenic Pentafluoride
____14. Bond angle between axial F As and the other axial F As in Arsenic
pentafluoride
Reading Reference:
Jespersen 7th Section 9.2; Note Figurer 9.4 page 408
Review Example 9.1 page 409
Try Practice Exercises
9.2/9.3 page 408
Study and Answer Review
Questions #9.3-#9.7 page 458
Work End of Chapter
Problems #9.73 -#9.90 pages 460-461 especially #9.81-9.82 and #9.83-9.84 page 461
Module 4iii Part N1: Geometry of Molecules 1 point
Use the dot/stick structures
on the Part L1 page to state the geometry of the molecules:
Steric Numbers 2, 3, or 4:
Trigonal Bent Linear Trigonal Planer Planer Trigonal Pyramidal Tetrahedral
Steric Numbers 5 or 6:
Trigonal-bipyramidal
Square Planer Seesaw T-shaped Octahedral Other
|
_______________1. BrF3 _______________2. XeO4 _______________3. SO2 _______________4. SO3
_______________5. XeF4 _______________6. BrF5 _______________7. AsF5 _______________8. SeF6 _______________9. SF4 _______________10. KrF2
_______________11. BrF3 _____________ Bonus C6H6 |
Benzene C6H6 |
Reading
Reference: Jespersen 7th Section 9.1/9.2 See Example 9.1 Page 409 See Steps page 413; See Example 9.4 Page415
Practice Exercise 9.1 page 406; Practice Exercises 9.2/9.3 Page 409; Practice
Exercises9.4-9.6 Page 415
Look
at End of Chapter Exercises #9,1-#9,7 page 458; Wrk
Review Problems #9.73-#9.82 page 460-1
Module 4iii Part O1: Polarity of Molecules 1 point
Sketch the 3D model
of the molecule, show all dipoles; then decide if the
molecule has a net dipole moment or not.
Write Polar or Nonpolar in each blank.
|
_______________1. BrF3 _______________2. XeO4 _______________3. SO2 _______________4. SO3
_______________5. XeF4 _______________6. BrF5 _______________7. AsF5 _______________8. SF6 _______________9. SF4 _______________10. KrF2
_______________11. BrF3 _____________ Bonus C6H6 |
Benzene C6H6 |
Reading Reference:
Section 8.6; Section
9.3
See Example 9.5 page 419 Work Practice Exercises 9.7/9.8 Page
420
Review Questions 9.8-9.15 pages 460-461
Try Problems 9.85-9.90 especially 9.89 and 9.90
Module 4iii - Part P1: Hybrid Orbitals of Molecules 1 point
Use the dot/stick structures
in the table to predict the hybrid orbitals that overlap to form the covalent
bond:
|
_____________1. BrF3 the sigma bond between
either Br-F _____________2. XeO4 the sigma bond between either Xe=O _____________2a. XeO4 the pi bond between either
Xe=O _____________3. SO2
the sigma bond between either
S=O _____________3a. SO2
the pi bond between either
S=O _____________4. SO3 the sigma bond between S=O _____________4a. SO2 the pi bond between S=O ____________ 5. XeF4
either sigma bond between any
Xe-F ____________6. BrF5 either single (sigma) bond between Br-F ____________7. AsF5
the sigma bond between any of
the As-F ____________8. SF6
the sigma bond between any of
the SeF6 ___________ 9.
SF4 either sigma bond between the As-F ___________ __10. KrF2
the sigma bond between either F-F _________ ____11. BrF3
either single (sigma) bond between Br-F _____________ 12. ClF5 either sigma bond between any Cl-F |
|
What is difference between a Sigma (σ) and a pi (π) bond? What is a delta(Δ) Bond
Reading Reference Jespersen 7th
: Section 9.5 and 9.6
Try Practice Exercise 9.11 and 9.12 page 427; Look at Example 9.6 page 428 and
9.7 page 430-431
Try Practice Exercise 9.14 and 9.15 Page 431 Study Example 9.8Page 432 Do Practice Exercises 9.16 and 9.17 page 433, the 9.20 and 9.21 page 440
Module
4iii - Part Q: Formal Charge
1 point
1. Using the method of the octet rule, the following structure was drawn:
|
|
Assign the formal charge to each atom in the Lewis
Structure Show the nonzero formal charges on the Lewis structure by Placing them in circles alongside the atoms |
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val
Electrons |
- |
Bonding
e1- 2 |
= |
Total |
|
S |
|
|
|
|
|
|
|
|
|
O- |
|
|
|
|
|
|
|
|
|
O= |
|
|
|
|
|
|
|
|
|
O- |
|
|
|
|
|
|
|
|
Sum of
the formal charges in the molecule = ______
2. Using the method of the formal charge, the following structure was drawn:
|
|
Assign the formal charge to each atom in the Lewis
Structure Show the nonzero formal charges on the Lewis structure by Placing them in circles alongside the atoms |
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val
Electrons |
- |
Bonding
e1- 2 |
= |
Total |
|
S |
|
|
|
|
|
|
|
|
|
O= |
|
|
|
|
|
|
|
|
|
O= |
|
|
|
|
|
|
|
|
|
O= |
|
|
|
|
|
|
|
|
Sum of the formal charges in the
molecule = ______
Explain why the
structure in #2 is more preferred than #1!
3.Using the method of the octet rule, the following structure was drawn:
|
|
Assign the formal charge to each atom in the Lewis
Structure Show the nonzero formal charges on the Lewis structure by Placing them in circles alongside the atoms |
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val
Electrons |
- |
Bonding
e1- 2 |
= |
Total |
|
S |
|
|
|
|
|
|
|
|
|
O= |
|
|
|
|
|
|
|
|
|
O= |
|
|
|
|
|
|
|
|
|
-O- |
|
|
|
|
|
|
|
|
|
H- |
|
|
|
|
|
|
|
|
|
-O- |
|
|
|
|
|
|
|
|
|
H- |
|
|
|
|
|
|
|
|
Sum of the formal charges in the
molecule = ______
4. Using the method of the octet rule, the following structure was drawn:
|
|
Assign the formal charge to each atom in the Lewis
Structure Show the nonzero formal charges on the Lewis structure by Placing them in circles alongside the atoms |
|
Formal Charge |
= |
Valence Electrons |
- |
Nonbonding Val
Electrons |
- |
Bonding
e1- 2 |
= |
Total |
|
S |
|
|
|
|
|
|
|
|
|
-O- |
|
|
|
|
|
|
|
|
|
H- |
|
|
|
|
|
|
|
|
|
-O |
|
|
|
|
|
|
|
|
|
-O- |
|
|
|
|
|
|
|
|
|
H- |
|
|
|
|
|
|
|
|
|
-O |
|
|
|
|
|
|
|
|
Sum of the formal charges in the
molecule = ______
Explain why the
structure in #4 is more preferred than #3!
Module
4iii - Part R: Resonance 1
point
1. Draw the Resonance Structures for Perchlorate Ion ClO41-
2. Draw the Resonance Structures for Phosphate Ion PO43-
3. Draw the Resonance Structures for Nitrite Ion NO21-
4. Draw the Resonance Structures for Benzene C6H6 ; show resonance hybrid
5. Draw the Resonance Structures for Carbonate Ion CO32-
Formal Charge References:
Reading Reference Jespersen 7th : Section 8.7 pages 377-384
Look at Calculating formal charges on an atom in a Lewis Structure page 379.
Formula:

Jespersens Formula:
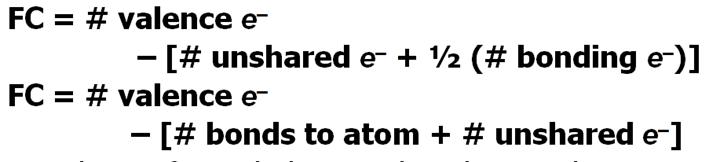
Look at Example 8.9 page 382-3
Try Practice Exercise 8.18; 8.19 and 8.20 page 383
Try Practice Exercise 8.21 and 8.22 Page 384
Do End of Chapter Questions #8.44-#8.47 page 397
Try Review Problems #8.105-#8.110 pages 399-400
Resonance References:
Reading Reference Jespersen 7th : Section 8.8
Look at Example 8.8 page 385
Try Practice Exercise 8.23; 8.24 and 8.25 page 386;
Note
the discussion on benzene on page 387
Answer
Review questions: #8.51-#8.54 page 397
Try
Review Problems: 8.113-8.118 page 400
Module
4iii - Part S: Sigma and Pi Bonds
1 point
Note Figures 9.30 and 9.31 formation of Sigma σ and Pi π Bonds !
- Sketch the hybrid orbitals for ethane (C2H4)
showing the 5 sigma bonds (see Figure 9.32)
- Sketch the p-p orbital overlap in ethane (figure 9.32 page 436)
- Sketch the hybrid orbitals for formaldehyde (CH2O )
showing the 3 sigma bonds (see Figure 9.34)
- Sketch the p-p orbital overlap in formaldehyde (CH2O
) (figure 9.34 page 438)
- Sketch the hybrid orbitals for Acetylene (C2H2 ) showing
the 3 sigma bonds (see Figure 9.35)
- Sketch the p-p orbital overlap in Acetylene (C2H2 ) (figure 9.35 page 439)
Reading
Reference Jespersen 7th: Section 9.6
Note
Brief Summary page 439.
Try
Practice Exercises 9.20 and 9.21 page 440
Answer
Review Questions # 9.30-#9.34 Page 409
Try
Review Problems #9.103-#9.110