CHM 2046C M-11ii-Chapter 16 Homework Packet Name:_________
|
Module 11ii: Acid/Base Equilibria Jespersen Chapter 16 Homework |
Possible |
Actual |
|
B.
Writing Equilibrium Constant Expressions
Section 16.3 |
1 |
|
|
C. Determination
of Kw from Kc ; Kb from
Ka & Kw ; Show Kb x Ka = Kw Lecture/16.3 |
1 |
|
|
E. Determination of pH of weak acids/bases
Problems Section 16.5 |
2 |
|
|
2 |
||
|
G.
Common Ion Effect Problem Section 16.7 |
2 |
|
|
1 |
||
|
I. Determination of pH of polyprotic
acids and/or Buffer Calculation Problem 16.8 |
2 |
|
|
J. Acid-Base Properties of Salts Section 16.6 |
1 |
|
|
K. Key Terms Chapter 16 p805-806 Summary |
1 |
|
|
Module 11ii Total: |
13 |
|
Part B: Equilibrium Constant Expressions of Acids and Bases 1 point
Write the appropriate equilibrium constant expressions for the following reactions they represent:
a. C5H5NH+1 + H2O çè C5H5N + H3O+1
Ka =
b. NH3 + H2O çè NH4 1+ + OH 1-
Kb =
c. H2CO3 + H2O ç=è HCO3 1- + H3O+1
Ka =
d. HCO3 1- + H2O ç==è CO3 2- + H3O+1
Ka2 =
e. C5H5N + H2O ç====è + OH 1- + C5H5N+1
Kb =
f. H2O +
H2O ç==è H3O 1+ +
OH 1-
Kw =
Reference: Jespersen Section 16.3
Try Practice 16.8; 16.9; 16.10; 16.11 Page 770;
Try Practice 16.12 and 16.13 page 771 Review Question 16.14-16.16.25 Pg 809
Answers: http://www.fccj.us/chm2046/SampleTest/46M11abAnswer.htm
CHM 2046C Module 11 Chapter 14 Homework Packet
Part C: Derivation of Kw Kh Kb and Ka Chapter 16 2 points
Write the ionization reaction of water, then develop the special K for water: Kw from the Kc
Write the hydrolysis reaction for Sodium acetate, NaC2H3O2 , when it is dissolved in water. Write the Kb expression for this reaction:
Write the ionization equilibrium expression for the Ka of acetic acid: HC2H3O2 and the Kw expression for water.
Derive the Kb expression from the Ka and the Kw expressions.
There is the following connection:
Ka x Kb = Kw
Using the ionization reaction of HCN in water, demonstrate
the development of the above formula from the Ka of HCN and its
conjugate base Kb of CN-
Reference: Section 16.3 Jespersen
Answers: http://www.fccj.us/chm2046/SampleTest/46M11cAnswer.htm
CHM 2046C Module 11ii –
Chapter 16 Homework Packet
Part E: Equilibria of Acids and Bases Calculations 2 points
Write the appropriate equilibrium constant expression for the following reaction:
E-1 (Determine Ka from Eq Concentrations_: A solution prepared from 0.055 mol of butanoic acid dissolved in sufficient water to give 1.0 L of solution has a pH of 2.72. Determine Ka for butanoic acid. The acid ionizes at according to the balanced equation.
CH3CH2CH2CO2H(aq) + H2O ó H3O+1 + CH3CH2
CH2CO2-1(aq)
|
|
CH3CH2 CH2CO2H |
H3O+1 |
CH3CH2 CH2CO2-1 |
|
Initial |
|
|
|
|
Change |
|
|
|
|
Equilibrium |
|
|
|
Reference: Section 16.4 Example 16.1 page 774; Example
16.2 Page 775
Try Practice Exercises 16.16; 16.17; 16.18 Page 776
Review Problems 16.75-16.88 Pages 811-812
E-2: Calculate Eq Concentrations from Ka and initial concentrations: What are the equilibrium concentrations of acetic acid, the acetate ion, and hydronium ion for a 0.10 M solution of acetic acid (Ka = 1.8 x 10-5)? What is the pH of the solution?
Ionization Reaction(you write):
Write the equilibrium
Reactions:
|
|
CH3CO2H |
H3O+1 |
CH3CO2-1 |
|
Initial |
|
|
|
|
Change |
|
|
|
|
Equilibrium |
|
|
|
Reference: Section 16.5 Example
16.3 Page 779
Try Practice 16.22; 16.23; 16.24 Pg 780; Review Problems 16.89-16.106 p812
Answers: http://www.fccj.us/chm2046/SampleTest/46M11efAnswer.htm
Part F: Hydrolysis Calculations 2 points
E1: What is the pH of the bleach solution which is 5.25% by weight Sodium hypochlorite, NaClO?
The Ka of Hypochlorous acid is 3.5 x 10-8
Write Hydrolysis reactions:
|
|
ClO 1- |
OH+1 |
HClO |
|
Initial |
|
|
|
|
Change |
|
|
|
|
Equilibrium |
|
|
|
E2: Calculate the pH of a 0.15M CH3NH3Cl
solution. For methylamine, CH3NH2, the Kb =
4.5 x 10-4. Problem 16.102
Write Hydrolysis reactions:
|
|
CH3NH3 1+ |
H1+ |
CH3NH2 |
|
Initial |
|
|
|
|
Change |
|
|
|
|
Equilibrium |
|
|
|
Reference: Section 16.6 Example 16.4 Pages 783-785
Try Practice Exercises 16.27; 16.28; 16.29; 16.30;
16.31 Page 785
Look at Review Questions 16.26-16.34 Page 809
Work Review Problems: 16.99-16.106 Page 812
Answers on Part E1 web Page:
http://www.fccj.us/chm2046/SampleTest/46M11efAnswer.htm
CHM 2046C Module 11ii
Homework Packet
Part G: Common Ion Solution Calculations 2 points
G1.A solution is prepared that is 1.50M HCOOH formic acid and 2.0 M Sodium formate. The Ka for formic acids is: 1.8 x 10-4
HCOOH + HOH <=====> H3O 1+ (aq) + HCOO1- (aq)
HCOONa + HOH <=====> Na 1+ (aq) + HCOO1- (aq)
What is the pH of the formic acid before adding the Sodium formate?
|
|
HCOOH |
H1+ |
HCOO1- |
|
Initial |
|
|
|
|
Change |
|
|
|
|
Equilibrium |
|
|
|
What is the pH of Sodium format solution before mixing?
|
|
HCOONa |
OH+1 |
HCOOH |
|
Initial |
|
|
|
|
Change |
|
|
|
|
Equilibrium |
|
|
|
What is the pH of the solution of formic acid and the sodium formate if in each case the volumes of the separate solutions are the same as the volume of the mixture with the concentrations that are listed above.
|
|
|
|
|
|
Initial |
|
|
|
|
Change |
|
|
|
|
Equilibrium |
|
|
|
Reference: Section 16.7 Example 16.5 Pages 787-788
Try Practice Exercises 16.34; 16.35; Page 788
Look at Review Questions 16.35-16.36 Page 809
Work Review Problems: 16.107-16.110 Page 812
Answers: http://www.fccj.us/chm2046/SampleTest/46M11gAnswer.htm
CHM 2046C Module 11ii
Homework Packet
G2. Preparing a Buffer
with a Desired pH:
How many grams of Sodium
acetate must be added to 1.0L of 0.15M Acetic Acid (pKa
4.74) to make a solution a buffer with a pH of 4.00? (RP 16.117)
Write the ionization
reactions of Acetic Acid and Sodium acetate:
Equilibrium Mixtures:
Use
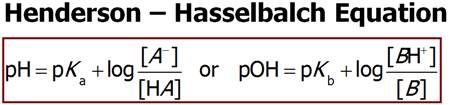
|
|
|
|
|
|
Initial |
|
|
|
|
Change |
|
|
|
|
Equilibrium |
|
|
|
Reference: Section 16.7 Example 16.6 Pages 789-790
Try Practice Exercises 16.36; 16.37; Page 790
Look at Review Question 16.37 Page 810
Work Review Problems: 16.114-16.120 Page 813
Part H: Discussion Questions Chapter 16 1 point
Explain the difference between an Arrhenius, Bronsted-Lowry, and Lewis Acid or Arrhenius, Bronsted-Lowry, and Lewis Bases?
Arrhenius Acid
Bronsted-Lowry Acid
Lewis Acid
Write equations to show how the HCO3 1- ion can act either as an acid or a base (likewise for HPO4 2- , HSO4 1- or H2PO4 1-):
HCO3 1-
HPO4 2-
H2PO4 1-
HSO4 1-
In ionization reactions of an
acid, base, or water one of the reactant molecules is water. But in the Ka,
Kb, and Kw expressions, the
concentration of water is not included in the calculation. Why?
What is the approximate molar
concentration of water at room temperature?
Why are salts from the
neutralization of a strong acid and a weak base test acidic with litmus paper
(Likewise a salt from the neutralization a weak acid and strong base test basic
with litmus paper)?
Why are salts from the
neutralization of a strong acid and a strong base test neutral (or a pH of 7.0)
with a pH meter?
Answers: http://www.fccj.us/chm2046/SampleTest/46M11hAnswer.htm
CHM 2046C Module 11ii Homework Packet
Part I: pH Polyprotic acid 2 points
Sulfurous acid, H2SO3,
is a weak acid capable of providing two hydrogen ions.
Ka1 = 1.2 x 10 -2 Ka2 = 6.2 x 10 -8
(a) Show both ionization reactions,
(b)write the equilibrium expressions for both)
(c) What is the pH of a 0.45 M solution of H2SO3
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(d) What is the equilibrium concentration of the sulfite ion, SO3 2- in the 0.45 M solution of H2SO3
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reference: Section 16.8 Example 16.8 Pages 794-795
Try Practice Exercises 16.40; 16.41; Page 796
Look at Review Question 16.37 Page 810
Work Review Problems: 16.121-16.126 Page 813
Answers: http://www.fccj.us/chm2046/SampleTest/46M11iAnswer.htm
CHM 2046C Module 11ii
Homework Packet
Part J: pH of Salts 1 point
Predict whether the following salts will test
A. acidic,
B. basic,
N neutral,
Not Enough Information to Predict
when dissolved in water.
Then show the hydrolysis reaction, if any
_____1. KCN(s) + HOH à
_____2. KNO3(s) + HOH à
_____3. NH4NO3(s) + HOH à
_____4. KC2H3O2 (s) + HOH à
_____ 5. NH4C2H3O2
(s) + HOH à
Reference: Section 16.6
Reference: Section 16.6 Example 16.4 Pages 783-785
Try Practice Exercises 16.27; 16.28; 16.29; 16.30;
16.31 Page 785
Look at Review Questions 16.26-16.34 Page 809
Work Review Problems: 16.99-16.106 Page 812
Answers: http://www.fccj.us/chm2046/SampleTest/46M11jAnswer.htm

Part K: Key Terms Chapter 16 1 point
1. __________________ – the equilibrium constant for the
ionization of an acid in water. The general form for a generic acid HA is
|
[H3O1+][A1-] |
|
Ka = --------------- |
|
[HA] |
2. __________________ – the product from a Lewis acid-base
reaction. In the acid-base adduct, the lone pair donated by the Lewis base is
shared with an anion in the Lewis acid.
3. __________________ – a substance that can behave either
as a Bronsted acid or a Bronsted
base.
4. __________________ – a substance that can behave as
either an acid or a base.
5. __________________ – a material that produces H+ ions in aqueous
solutions.
6. __________________ – a material that produces OH – ions in aqueous
solutions.
7. __________________
– a process in which
molecules of the same material react with each other to produce ions. The
_________ of water produced both hydronium and
hydroxide ions.
8. __________________– the equilibrium constant for the
ionization of a base in water. The general form for a generic base B is
|
[BH1+][OH1-] |
|
Kb = --------------- |
|
[B] |
9. __________________ – a material that can donate a proton
to another substance
10. _________________ – a material that can accept a proton
from another substance
11. _________________ – a metal ion that has Lewis bases joined to it by
coordinate covalent bonds.
12. _________________ – two species that differ from each
other by the presence of one H +
ion.
13. ____________________ - the attraction of electrons from
adjacent bonds by an electronegative atom.
14. _________________ –
in a aqueous solution.
Kw = [H3O +][OH -]
15. _________________ – a material that accepts an electron pair in a chemical
reaction
16. _________________ - A material that donates an electron pair in a chemical reaction
17. _________________ – a material that is capable of
donating one proton per formula unit of the acid
18. _________________ – a material that is capable of
accepting only one proton per formula unit of the acid
19. _________________
– the negative
logarithm of the hydronium ion concentration
(pH = - log [H3O+])
20. _________________– the negative logarithm of the hydroxide ion concentration
(pOH
= - log [OH-])
21. _________________
– a material capable of donating more than one proton per formula unit of the
acid
22. _________________
– a material capable of accepting more than one proton per formula unit of the
base
23. _________________ - is the reaction of a substance with water
Reference: Chapter 16 Summary Pages 805-806