CHM
2046C Module-4ii Homework Packet Name: ____________________
Please
complete the following homework packet before you actually attempt the pretest
before or after class each day. This is homework, not the pretest. You attempt
it before you take the exam. You grade it. This completed packet is due the day
of the exam.
B._____ (05) Lewis Dot/Stick Structure via Octet Rule Steric#s 2-3-4 Sect 8.3/8.7Answer
E._____ (01)
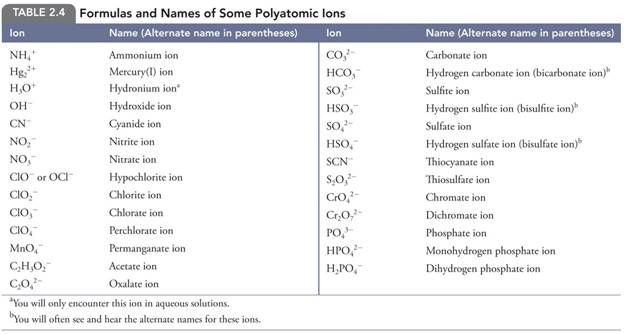
Polyatomic Ions† Table
2.4 Page 85
L.† ____ (01)
Bond
Angles/Bond Lengths Steric#s 2-3-4
Section 9.2 Answers
N. ____† (01) Geometry of Molecules- Steric#s 2-3-4 Section 9.1-9.2† Answers
O. ____† (01) Polarity of Molecules- Steri #s 2-3-4 Section 8.6, 9.3 Answers
P. ____†† (01) Hybrid Orbital Recognition Steric#s 2-3-4-Sect 9.5-6 Answers
______(10) Total
Module
Four II: Part B Dot Structures of Molecules Review†† 05 points
Choose
Two from each question. Use the simple octet rule (but
you may use formal charge method for a more accurate drawing-Required in M4iii
B1). Using
a periodic chart/octetrule draw the electron dot/stick structures of the
following molecules:
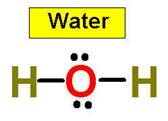
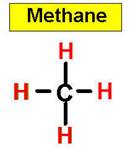
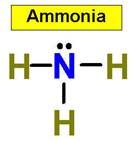
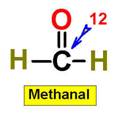
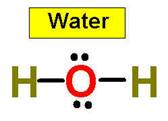
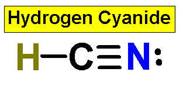
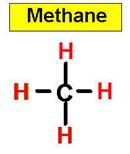
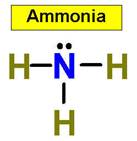
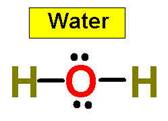
1.† NH3†† CH4††† H2O2†††† H2O†† HCl††
H2†† O2†† N2†
2.† H2SO4 H2SO3† H3PO4
†H3PO3† HClO4†† HClO3† HClO2†† HClO
3.†††† HNO3†† H2CO3† HNO2† †††††††††††† †
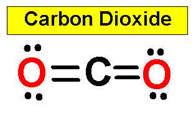
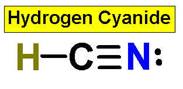
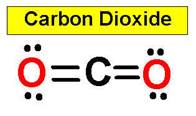
4.††††† CO2††† HCN†††
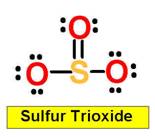
SiO2†† SO3††
5.†† HC2H3O2†††† H2C2O4††† ††††† †HCHO2†††††† ††
carbon to carbon by single covalent bond
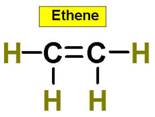
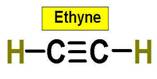
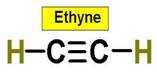
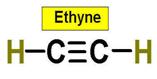
6.††††† C2H4††† C2H2†† ††C3H8† †††C2H6
†††††† †††††† bond carbons to carbon
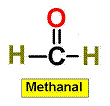
7.††† CH2O†† †††††††††††CH3COCH3†† †††††
††††††††††††† carbon
to carbons by single covalent bonds above
8. †CH3OCH3††† †††††††††††††††††††††††††††††††††††CHONH2† †††††††††††††††††††††††††††
†††††† oxygen
separates †††††††††††††††††††††††††††††††††O & N both ††††††††††††††††††††††††††††††††
††††† ††the carbons††††† †††††† ††††††††††††††††††††††††††††††bond to C †††††††††††††††††††††††††††††††††
††††††††††††††††††††††††††††††† †††††††††††††††††††††††††††††
9. †CH2NH2COOH†††††††††††††††††††††† CH3CHNH2COOH††††††††††
††††††† carbon to
carbons by single covalent bonds† -NH2
hooks to #2 carbon in both above
10.† CH3COOCH2CH3†††††††††††††††††††††††††††††††††††††† CHOOCH3
††††††††††† carbon to
carbons by single covalent bonds†† †††††††(-CH3 also hooks to an oxygen
above)
†††††††††† (-CH2CH3 also
hooks to an oxygen above)
Reading
Reference: Sections 8.5 Octet Rule and 8.7 Formal Charge (page 374)
Reference:
B.†† Dot Structures of Covalent Compounds Section
2.10, 6.6, 7.1, 7.5, 7.6
Answers: http://www.fccj.us/chm2045/SampleTest/45M4bAnswers.htm
††† Required
List of Polyatomic Ions 0 Points
|
1 |
IA |
IIA |
|
|
|
|
H |
|
|
|
|
|
IIIa |
IVA |
VA |
VIA |
VIIA |
He |
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
B |
C* |
N |
† |
† |
Ne |
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Si |
P |
S |
Cl |
Ar |
|
4 |
|
|
|
|
V |
Cr |
Mn |
Fe |
|
|
|
|
|
|
As |
Se |
Br |
Kr |
|
5 |
|
|
|
|
|
Mo |
|
|
|
|
|
|
|
Sn |
Sb |
Te |
I |
Xe |
|
6 |
|
|
|
|
|
W |
|
|
|
|
|
|
|
Pb |
|
|
|
Rn |
|
7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acetate††††††††††† ___________ Ammonium† †† ___________ Antimonate† †† ___________ Antimonite††† †† ___________ Arsenate†††††† †† ___________ Arsenite††††††† †† ___________ Azide††††††††††††† †† ___________ Bicarbonate††††† ___________ Borate†††††††††††††† ___________ Bromate††††††† †††† ___________ Bromite†††††††† †††† ___________ Carbide†††††††††††† __________ Carbonate†††††††† __________ Carbonite††††††††† __________ Chlorate††††††††††† __________ Chlorite†††††††††††† __________ Chromate††††††††† __________ Cyanate†††††††††††† _________ Cyanide†††††††††††† _________ Dichromate†††††† __________ Dihydrogen††† Arsenate††
__________ Dinhydrogen
Phophate†† __________ Dihydrogen†† Phosphite†
__________ Ferrate†††††††††††††††††††††††††††††
___________††††††††††† Hydrogen
carbonate††††††† ___________ Hydrogen
arsenate††††††††† ___________ Hydrogen
phosphate††††† ___________ Hydrogen
phosphite†††††† __________ Hydrogen
sulfate††††††††††† __________ Hydrogen
sulfite†††††††††††† __________ Hydronium††††††††††††††††††††† __________ Hydroxide†††††††††††††††††††††† ___________ |
Hypobromite†††††††††††††††† __________ Hypochlorite†††††††††††††††† __________ Hypoiodite†††††††††††††††††††† __________ Hypophosphite ††††††††††††__________ Hyposulfite†††††††††††††††††† ___________ Periodate†††††††††††††††††††††† __________ Iodate†††††††††††††††††††††††††††† __________ Iodite††††††††††††††††††††††††††††† __________ Mercury (I)†††††††††††††††††††† __________ Manganate†† ††††††††††††††††††__________ Molybdate††††††††††††††††††††† _________ Nitrate†††††††††††††††††††††††††††† __________ Nitrite††††††††††††††††††††††††††††† __________ Oxalate†††††††††††††††††††††††††† __________ Perbromate††††††††††††††††††† __________ Perchlorate††††††††††††††††††† __________ Permanganate†††††††††††††† __________ Phosphate†††††††††††††††††††† __________ Phosphite††††††††††††††††††††† __________ Plumbate†††††††††††††††††††††† __________ Plumbite††††††††††††††††††††††† __________ Selenate†††† †††††††††††††††††††___________ Selenite†††††††††††††††††††††††† ___________ Silicate††††††††††††††††††††††††† ___________ Stannate†††††††††††††††††††††† ___________ Stannite††††††††††††††††††††††† ___________ Sulfate††††††††††††††††††††††††† ___________ Sulfite†††††††††††††††††††††††††† ___________ Tellurate†††††††††††††††††††††† ___________ Thiocyanate†††††††††††††††† ___________ Tungstate†††††††††††††††††††† ___________ Vanadate††††††††††††††††††††† ___________ |
|
Reference Online Homework: Polyatomic Ions-Section 2.12 Answers e†††††††††††††††††††††††††††††††††††††††††††††
http://www.northcampus.net/Nomenclature/PolyatomicIonFormula/ProgressivePolyatomicIonFormula.html
††††††††††† Required List of Polyatomic Ions
|
1 |
IA |
IIA |
|
|
|
|
H |
|
|
|
|
|
IIIa |
IVA |
VA |
VIA |
VIIA |
He |
|
2 |
|
|
|
|
|
|
|
|
|
|
|
|
B |
C* |
N |
† |
† |
Ne |
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Si |
P |
S |
Cl |
Ar |
|
4 |
|
|
|
|
V |
Cr |
Mn |
Fe |
|
|
|
|
|
|
As |
Se |
Br |
Kr |
|
5 |
|
|
|
|
|
Mo |
|
|
|
|
|
|
|
Sn |
Sb |
Te |
I |
Xe |
|
6 |
|
|
|
|
|
W |
|
|
|
|
|
|
|
Pb |
|
|
|
Rn |
|
7 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following Polyatomic Ions
should be in you chemistry vocabulary:
|
Acetate CH3COO−
†††††††††
or C2H3O2− Ammonium††††† NH4+ Antimonate††††† SbO43− Antimonite†††††† SbO33− Arsenate†††††††††† AsO43− Arsenite†††††††††† AsO33− Bicarbonate†††† HCO3− Borate††††††††††††† BO33− Bromate†††††††††† BrO3− Bromite††††††††††† BrO2− Carbide††††††††††††
C22− Carbonate†††††††† CO32− Carbonite†††††††††
CO22− Chlorate††† †††††††††ClO3− Chlorite†††††††††††††
ClO2− Chromate†††††††††† CrO42− Cyanate††††††††††††† OCN− Cyanide††††††††††††† CN− Dichromate†††††††
Cr2O72− |
Dihydrogen †††††††
Arsenate†† ††††††††††††H2AsO4− Dinhydrogen ††††††
Phophate††† †††††††††††H2PO4− Dihydrogen ††††††
Phosphite†† †††††††††††H2PO3 Hydrogen
carbonate†† †HCO3− Hydrogen
arsenate†† †††HAsO42− Hydrogen
phosphate† †HPO42− Hydrogen
phosphite††† HPO32− Hydrogen
sulfate††††††††† HSO4− Hydrogen
sulfite†††††††††† HSO3− Hydronium††††††††††††††††††† H3O+ Hydroxide†††††††††††††††††††† OH− Hypobromite†††††††††††††††† BrO− Hypochlorite†††††††††††††††† ClO− Hypoiodite†††††††††††††††††††† IO− Hypophosphite†††††††††††† PO23− Hyposulfite†††††††††††††††††† SO22− |
Iodate††††††††††††††††† IO3− Iodite†††††††††††††††† ††IO2− Manganate† †††††††MnO42- Mercury (I)††††††††††
Hg22+ Nitrate††††††††††††††††† NO3− Nitrite†††††††††††††††††† NO2− Perbromate††††††††
BrO4− Perchlorate††††††††
ClO4− Periodate†††††††††††
†IO4− Permanganate† MnO4− Phosphate†††††††† PO43− Phosphite††† ††††††PO33− Plumbate†††††††††† PbO32− Selenate††††††† ††††SeO42− Selenite††††††††
††††SeO32− Silicate†††††††††† †††SiO32− Stanate††††††††††††† SnO32− Sulfate††††††††††† †††SO42− Sulfite††††††††††††
†††SO32− Tungstate††††††† ††WO42− |
Bonus 1 point Each: Using a current CRC Handbook of Chemistry/Physics find a polyatomic ion list in an inorganic compound that is not on the list below:
The Complete Polyatomic Ions List http://www.fccj.us/PolyatomicIons/CompletePolyatomicIonList.htm
Taylorís ĺ Rule: http://www.fccj.us/PolyatomicIons/Taylor34OxygenRuleHandout.htm
Taylorís Charge Rule: http://www.fccj.us/PolyatomicIons/TaylorChargeRuleHandout.htm
National Publication† 2YC3 Newsletter (2014 vol iii)
http://www.2yc3.org/Newsletters/2014/2014iii.pdf
Jespersenís Common Polyatomic Ions
CHM 2046C Progressive
Polyatomic Ions Jespersen ††††††††††††††††††††††††††††††††††††††††††1
Point
†††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††
†Name:_____________________
Write the formula and the charge for the following
polyatomic ions: Jespersen(Table 2.4
page 85)
†††††††
|
Name |
Formula with charge |
Name |
Formula with charge |
|
Ammonium |
|
Sulfite |
|
|
Mercury(I) |
|
Hydrogen Sulfite |
|
|
Hydronium |
|
Sulfate |
|
|
Cyanide |
|
Hydrogen Sulfate |
|
|
Nitrite |
|
Thiocyanate |
|
|
Nitrate |
|
Thiosulfate |
|
|
Hypochlorite |
|
Chromate |
|
|
Chlorite |
|
Dichromate |
|
|
Chlorate |
|
Phosphate |
|
|
Perchlorate |
|
Monohydrogen Phosphate |
|
|
Permanganate |
|
Dihydrogen Phosphate |
|
|
Acetate |
|
Hydrogen
Carbonate |
|
|
Oxalate |
|
Carbonate |
|
Module
Four II: Part L Bond Angles†††††††††† †††††††††††††††††††††††††1 point
What
is the bond Angle in the following structures:
†
___1. 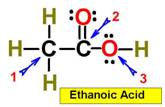 †††___4.
†††___4.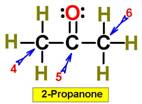
___2.††††††††††††††††††††††††††††††††††††††† †††† ___5.
___3.††††††††††††††††††††††††††††††††††††††† †††† ___6.
___7. 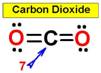 †††††___8.
†††††___8. 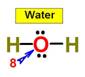 ____9.
____9.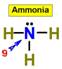
___10. 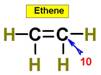 ††††††____11.
††††††____11. 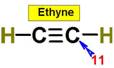
___12. 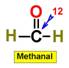 ††___13.
††___13. 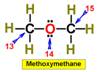 †___16.
†___16.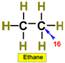
††††††††††††††††††††††† ††††† ___14.
††††††††††††††††††††††† ††††† ___15.
___17.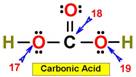 †††††††††††††††††† ___20.
†††††††††††††††††† ___20.
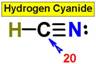
††††††††††††††††††† ____18.†† _____19.
Bonus:
†___21. 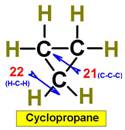 ††___23.
††___23. 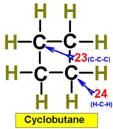
___22.††††††††††††††††††††††††† †††††††† ___24.
Steric
Numbers do not predict bond angles within rings of carbons
Reading
Reference: Jespersen 7th Section 9.2;
Work
End of Chapter Problems #9.83-9.84 page 461
Module Four II - Part N: Geometry of Molecules†††† †††††††††††††††††††††††1 point
Use the dot/stick structures on the Part L page to state the geometry of the molecules:
Steric # = 2, 3,or 4:
Bent††††† Linear††††† Trigonal Planer† Planer††† Trigonal Pyramidal††††† Tetrahedral
Steric # = 5 or 6†
Trigonal-bipyramidal††††† Square Planer†††† Seesaw††††† T-shaped††† Octahedral
|
_____________1.†
H2O _____________2.†
CO2 _____________3.†
C2H4 _____________4.†
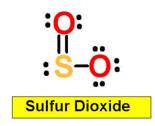
SO2 _____________5.†
SO3 _____________6.†
HCN _____________7.†
CH4 _____________8.†
NH3 _____________9.†
CH2O _____________10. C2H2 †______________ 11. PF5
† _____________† 12. SF6 |
|
Reading
Reference: Jespersen 7th Section 9.1/9.2† See Example 9.1 Page 409† See Steps page 413; See Example 9.4 Page415
Practice Exercise 9.1 page 406; Practice Exercises 9.2/9.3 Page 409; Practice
Exercises9.4-9.6 Page 415
Look
at End of Chapter Exercises #9,1-#9,7 page 458; Wrk
Review Problems #9.73-#9.82 page 460-1
Module Four II:† Part O: Polarity of Molecules††††† 1 point
Draw the dot structure for the following compounds, then state whether the molecule is polar or nonpolar:
|
_____________1.† H2O _____________2.† CO2 __________ _ _3.†
C2H4 _______ ______4.†
SO2 __________ ___5.†
SO3 ________ _____6.†
HCN _________ ____7.†
CH4 ________ _____8.†
NH3 ______________9.†
CH2O ______________10. C2H2 †_______________ 11. PF5
† ______________† 12. SF6 |
|
Reading Reference: Section 8.6;† Section 9.3
See
Example 9.5 page 419†
Work Practice Exercises 9.7/9.8 Page 420
Module Four II - Part P: Hybrid Orbitals of Molecules††††† †††††††1 points
Use the dot/stick structures
in the table to predict the hybrid orbitals that overlap to form the covalent
bond:
|
_____________1.† H2O†† the sigma bond ††††††† ††††††††††††††††††††††between either† H-O _____________2.† CO2† the sigma bond ††††††††††††††††††††††††††††† between either
C=O _____________2a.† CO2††††† the pi bond †††††††††††††††††††††††††††††† between either
C=O _____________3.††† C2H4†† the sigma bond †††††††††††††††††††††††††††††† between the
two carbons _____________3a.† C2H4†† the pi bond †††††††††††††††††††††††††††††† between the
two carbons _____________4.††† SO2† the sigma bond †††††††††††††††††††††††††††††† between S=O _____________4a.† SO2 †the pi bond †††††††††††††††††††††††††††††† between S=O ____________4b.† SO2† the sigma bond †††††††††††††††††††††††††††††† between S-O ____________ 5.††† SO3††
either sigma bond †††††††††††††††††††††††††††††† between S-O ____________ 5a.† SO3††
the sigma bond ††††††††††††††††††††††††††††† between S=O ____________ 5b.† SO3†† the pi bond ††††††††††††††††††††††††††††† between S=O ____________6.††† HCN†
the single (sigma) ††††††††††††††††††††††††††††† bond between
H-C ____________6a.† HCN†
the sigma bond †† †††††††††††††††††††††††††††between C=N ____________6b.† HCN†
either pi bond ††††††††††††††††††††††††††††† between C=N ____________7.† CH4†
the sigma bond ††††††††††††††††††††††††††††† between any of
the H-C ____________8.† NH3†
the sigma bond †††††††††† †††††††††††††††††††between any of the H-N ___________† 9.††
CH2O† either sigma ††††††††††††††††††††††††††††† bond between
the H-C ____________9a.† CH2O† the sigma†
bond ††††††††††††††††††††††††††††† between the C=O ____________9b.† CH2O† the pi†
bond † ††††††††††††††††††††††††††††between the C=O ___________ __10.†† C2H2†† the sigma bond ††††††††††††††††††††††††††††† between the C=C _________ ____10a. C2H2†† either pi bond ††††††††††††††††††††††††††† between the C=C _____________ 10b. C2H2† the sigma † ††††††††††††††††††bond between any of the H-C |
.
|
What is difference between a Sigma (σ) and a pi (π) bond? What is a delta(Δ) Bond
Reading Reference Jespersen 7th
: Section 9.5 and 9.6
Try Practice Exercise 9.11 and 9.12 page 427; Look at Example 9.6 page 428 and
9.7 page 430-431
Try Practice Exercise 9.14 and 9.15 Page 431† Study Example
9.8Page 432 Do Practice Exercises 9.16 and 9.17 page 433, the 9.20 and 9.21
page 440