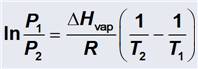CHM
2046C Pretest Homework Packet
Name: ____________________
Please
complete the following pretest homework sections before you attempt the exam.
This is homework. You grade it. This completed packet is due the day of the
exam. You must show all work. Any mathematical type
problem application must show work. No credit for just writing the answer. No
credit for a section if the sample problem is shown and you leave any
additional problems which do not show the answer blank.
Module Seven Part II: Intermolecular Forces &
Liquids/Solids
______(01) P. Phase Diagrams
______(01) Q. Intermolecular Forces
______(01) Q1: Intermolecular Forces Physical Properties
______(02) R. Enthalpy Change with Phase
Change
______(01) S. Discussion Questions-Chapter11
Jespersen
______(02) U. Clausius-Clapeyron Equation Calculation
______(02) V. Vapor Pressure Calculation
_____ (00)
T. Key Terms-Chapter 11
----------------
______(10) Total M7ii Homework Packet
Part
P Phase Diagrams 01 points
Identify
the points labeled on the
Phase Diagram of
Water
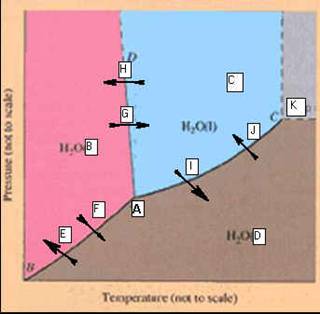
_____________________A.
_____________________B. _____________________H
_____________________C. _____________________I
_____________________D. _____________________J
_____________________E. _____________________K
_____________________F
_____________________G
Phase Diagram
for Carbon Dioxide
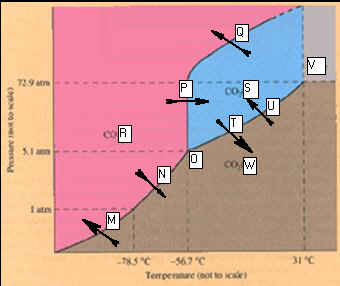
_____________________M.
_____________________N.
_____________________O.
_____________________P.
_____________________Q.
_____________________R
_____________________S
____________________ T.
____________________
U.
____________________
V.
____________________
W.
Reading Reference:
Jespersen 7th Section 11.7 pages 541-543
Phase Diagram Question 11.57-11.65 p 568
See Examples 11.3/11.4 p 543
Work Practice Exercises 11.12-11.14 Page
544
Extra Exercises 11.111-11.114 page 571
Module 7II Q
Intermolecular Forces 1
Point
Describe the different type of
interparticle forces that can occur between atoms,
molecules, and ions.
Distinguish the forces called
intermolecular forces.
What forces are referred to as
van der Waals forces?
Draw a flow chart or diagram
to summarize these intermolecular forces and show an example.
(Use Table 11.3 page 5.24)
|
Type of Interaction |
Factors Responsible For Interaction |
Approximate Energy (kJ/mol) |
Example |
|
Ion-dipole |
|
|
|
|
Dipole-dipole |
|
|
|
|
Hydrogen
Bonding, X—H…:Y |
|
|
|
|
Dipole-induced
dipole |
|
|
|
|
Induced
dipole-induced dipole ( |
|
|
|
Reading Reference Jespersen 7th Section 11.1
See Example 11.1 Page 524
Module 7II Q1
Intermolecular Forces 1
Point
1.
Define:
Compressibility
Diffusion
Surface Tension
Wetting
Viscosity
2.
Explain why when you use an
alcohol swab before receiving a shot the skin feels cool?
Reading Reference: Jespersen 7th Section
11.2 pages 525-531
Practice Exercise 11.4 page 531
End of Chapter Exercises Questions
11.12-11.29 pages 566-7
Part
R: Enthalpy Change with Phase Changes 2 points
You put
2.50 L of water in a soup pot at 100 oC and the water slowly evaporates. How
much heat must have been supplied to evaporate all the water from the pot.
Δ Hvap
of water is +40.7 kJ/mole and
the density of water at 100oC
is 0.958 g/mL
Suppose 60.0 g of water at 75oC is added to 120
grams of ice at 0oC, How many grams of ice will melt and
what will be the final temperature of the mixture?
he molar heat of fusion of water 6.01 kJ/mol and the
specific heat of water is 4.18 J/g oC
Reading Reference Jespersen 7th Section 11.3 and Section 11.6
See Worked Example 11.2 Page 539
Work Practice Exercises 11.10 and 11.11
Page 540
End of Chapter Exercises Questions 11.107-11.110 Pages 570-1
Part
S: Discussion Questions 1 point
1.a. What is the Hydrogen Bond?
1.b. Describe the requirements for
the Hydrogen Bond..
1.c. Draw a hydrogen bond between a
water molecule and an ethanol (CH3CH2OH) molecule.
2a.
Describe the process of dynamic equilibrium that exists between a liquid and
its vapor in a closed container.
2.b. Why does a system in an open
container never reach equilibrium?
3.a. What is the critical point?
3.b. Draw a vapor pressure curve for
water and demonstrate the critical points
4.a. Describe the phenomenon of
surface tension.
4.b. does water climb up the sides
of glassware, while Mercury would not? Sketch/label tubing to show each
Part U: Clausius-Clapeyron Equation Calculation 2 points
The Clausius-Clapeyron Equation is:
|
|
Calculate: ΔHovap
- Diethyl ether is a volatile, highly flammable organic liquid that
today is mainl used as a solvent. Calculate the enthalpy of vaporization (ΔHovap ) of diethyl ether, (C2H5)2O.
Diethyl ether has vapor pressures of 57.0 torre and 534 torre at -22.8oC and 25.4oC respectively. R = 8.3145 J/K∙mol
Addition End of chapter exercises: #11.119 and
11.120 Page 571 (ΔHovap)
Calculate: P2
- The vapor pressure of diethyl ether is 401 torre
at 18oC, and the molar heat of vaporization (ΔHovap) is 26kJ/mole , Calculate the vapor
pressure at 32oC!
Calculate: T2
- At what temperature would diethyl ether have a vaport
pressure of 250 torre?
Addition End of chapter
exercises: #11.117 and 11.118 Page 571 (P2)
Reading
Reference: Jespersen 7th Section11.9 Pages 547-549
See Example 11.5 pages 548-549
Try Practice Exercises 11.17/11.18 page
549
Part V: Vapor
Pressure Calculation 2 point
If 0.50 g of pure water is
sealed in an evacuated 5.0 L flask and the whole assembly is heated to 60oC,
will the pressure be equal to or less than the equilibrium vapor pressure at
this temperature?
If less what will be the pressure in the
flask?
What if you used 2.0 g of water? Under either
set of conditions, is any liquid water left in the flask, or does all the water
evaporate? (The equilibrium vapor pressure of water at 60oC is 149.4
torr)
Addition End of chapter exercises:
#11.36-11.41 pages 567-568
Reading
Reference: Jespersen 7th Section 11.4 Pages 533-535
No Sample Exercises in Jespersen 7th text or additional problems
Module
Seven Part T: Key Terms Chapter 11 0
Points
Fill in
the blank with the word(s) which best fit the description:
__________________1.
The heat required to convert solid to a liquid at its melting point.
___________________2. The heat required to convert
liquid to a gas at its boiling point.
___________________3. A pressure-temperature plot that
shows the conditions under which a
substances exits as a solid, liquid, and a gas.
___________________4. Refers to the
conversion of a gas (vapor) to a liquid.
___________________5. The unique temperature and
pressure at which the solid, liquid, and
gaseous phases of a substance exists.
___________________6.
Is the passage of molecules of a liquid to the gaseous state.
___________________7. The
temperature at which a solid melts.
___________________8. The highest temperature at which
a liquid can coexist in equilibrium
with its vapor.
___________________9. The temperature at which a
liquid’s vapor pressure becomes equal to
the prevailing atmospheric pressure.
___________________10. Pressure exerted by the vapor in
dynamic equilibrium with a liquid.
at a constant
temperature
Reading Reference: Chapter 11 Summary pages 563-565