CHM 2046C Name: ________________
Module 13 Paper and Pencil Homework Packet
|
|
|
|
|
|
Possible |
Actual |
||
|
5 |
|||
|
5 |
|||
|
5 |
|||
|
5 |
|||
|
5 |
|||
|
5 |
|||
|
G. Calculation Free
Energy from Standard Free Energy of Formation |
5 |
||
|
H.Calculation of Equilibrium Constant from Standard Free Energies
of Formation |
5 |
||
|
5 |
|||
|
Module Thirteen
Total: |
45 |
|
Part A. Laws of
Thermodynamic 5 points
1. Describe the
difference between Thermodynamics and Kinetics of a
chemical reaction.
- What
two components drive a naturally occurring process? Explain their
relationship to a spontaneous process.
3. What do you understand by the word entropy?
4. State the First Law of Thermodynamics.
.
5. State the Second Law of Thermodynamics.
.
6. State the Third Law of Thermodynamics.
Page
2 M-13 Homework Packet
Part B Entropy: Disorder and Spontaneity 5 points
1. What six
types of processes or reactions lead to an increase in entropy?
2. How are
entropy, enthalpy, and the spontaneity of a reaction related?
The reaction is spontaneous
if
___________________________.
The possibilities are:
|
∆Hosys |
∆Sosys |
Result |
|
(exothermic) |
|
|
|
(exothermic) |
|
|
|
(endothermic) |
|
|
|
(endothermic) |
|
|
3. Which of the
following processes are spontaneous and which are nonspontaneous:
(a) diffusion of perfume molecules from one side of the room to
the other.
(b) Heat flow from a
cold piece of metal (2oC)to hot water(70oC)
when the cold metal is dropped into the hot water.
(c) Decomposition of
rust (Fe2O3∙H2O) to iron metal, oxygen
gas, and water.
(d) Decomposition of
solid CaCO3 to solid CaO and gaseous CO2 at 25oC
and 1 atm pressure (Kp = 1.4 x 10 -23)
4. Predict the
sign of ∆S in the system for each of the following processes:
(a) CO2 (s) --->
CO2 (g) (sublimation
of dry ice)
(b) CaSO4(s) ---> CaO(s) + SO2(g)
(c)
N2(g)
+ 3 H2 (g) ---> 2 NH3
(g)
(d) I2(s) ---> I2 (aq)
(dissolution of iodine in water)
Page
3 M-13 Homework Packet
Part C: Calculating Standard Entropy of Reaction 5 points
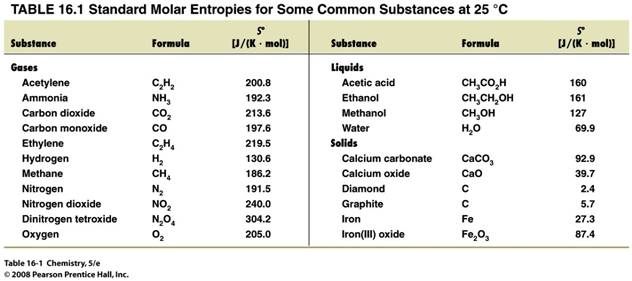
Calculate the standard entropy of
reaction at 25oC for the Haber Process of
ammonia:
N2 (g) +
3 H2 (g) ΰ 2 NH3 (g)
Page
4 M-13 Homework Packet
Part D: Derivation of Gibbs Free Energy Change & Discussion: 5 points
The quantity called the Gibbs free-energy change (∆G),
∆G
= ∆H - T∆S, determines whether a chemical or
physical process will occur spontaneously. Fill in the following:
∆G < 0 Process
is _______________________________________________
∆G = 0 Process
is _______________________________________________
∆G > 0 Process
is _______________________________________________
This quantity: ∆G = ∆H - T∆S
is derived from the following equation:
∆Souniverse
= ∆Sosystem + ∆Sosurroundings
Show this derivation
with stepwise explanations/comments:
Page
5 M-13 Homework Packet
Part E: Reaction Spontaneity
Calculation: 5 points
The quantity called the Gibbs free-energy change (∆G),
∆G = ∆H - T∆S, determines whether a chemical or physical process will occur spontaneously. Iron metal can be produced by reducing Iron III oxide (rust) with Hydrogen:
Fe2O3 (s) + 3 H2 (g) ---> 2 Fe (s) + 3 H2O (g) ∆Ho =
+98.8 kJ;
∆So = +341.5 kJ/K
(a) Is this reaction spontaneous under standard state
conditions at 25oC?
At what temperature will the
reaction be become spontaneous?
Page
6 M-13 Homework Packet
Part F: Calculation ∆Go from ∆Ho and ∆So 5 points
Using the quantity called the Standard
Gibbs free-energy change (∆Go),
∆Go = ∆Ho - T∆So,
perform the following calculations using values in Appendix B:
Iron metal is produced commercially by reducing Iron III oxide in iron ore with
Carbon monoxide:
Fe2O3
(s) + 3 CO (g) ---> 2 Fe (s) + 3 CO2 (g)
(b) Calculate the standard free-energy change for this
reaction at 25oC?
(c) Is the reaction spontaneous under standard conditions
at 25oC?
(d) Does the reverse reaction become spontaneous at higher
temperature? Explain.
Page
7 M-13 Homework Packet
Part G: Calculation of ∆Go Values from ∆Gof
Values 5
points
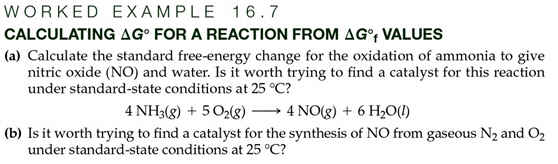
Page
8 M-13 Homework Packet
Part H: Calculation of an Equilibrium Constant from ∆Go Values
5 points
See
Table 16.4 page 673; and worked example 16.9 page 673:
Methanol (CH3OH,
an important alcohol used in the manufacture of adhesives, fibers, and
plastics, is synthesized industrially by the reaction:
CO (g) +
2 H2 (g) ↔ CH3OH (g)
Use the
thermodynamic data in Appendix B (values below) to calculate the equilibrium
constant for the reaction at 25oC.
Additional Values from Appendix B:
∆Gof
CO =
51.3 kJ/mol
∆Hof
CO =
-110.5 kJ/mol
So
CO =
197.6 J/K∙mol
So H2 =
130.6 J/K∙mol
∆Gof
CH3OH
= -166.4 kJ/mol
∆Hof
CH3OH= -201.2 kJ/mol
So
CH3OH= 238 J/K∙mol
Page
9 M-13 Homework Packet
Part
K: Chapter 16 Key Terms 5
points
1. _____________ the amount of molecular randomness in a
system
2. _________________ The total
internal energy of an isolated system is constant.
3. _________________ . A thermodynamic state function relating
enthalpy, temperature, and entropy
4. ___________________ - In any spontaneous
process, the total entropy of a system and its surroundings always increases..
5. ___________________ a process that proceeds
on its own without any continuous external influence
6. ___________________ entropy change under
standard state conditions
7. __________________-:The free-energy
change for formation of
1 mol of a substance in
the standard state from the most stable form of the constituent elements in
their standard states. :
8. ___________________ the entropy of one mole
of a pure substance at 1 atm pressure and a specific temperature, usually 25oC
for a gas, 1 M concentration for a solution
9. ___________________ The most stable
form of an element or compound in the physical state in which it exists at 1
bar and the specific temperature. `.
10. _________________ the study of the interconversion of heat and
other forms of energy
11. ___________________ The entropy of
a perfectly ordered crystalline substance at Zero Kelvin (0 K) is Zero
Review Terms:
12. __________________ the heat
change in a reaction or process at constant pressure ∆H =
∆E + P∆V
13. __________________ The
substance being evaluated for energy content in a thermodynamic process.
14. __________________
everything outside the system in a thermodynamic process
15. __________________- a reaction
in which heat is evolved and the temperature of the surroundings rises
16. __________________- a
reaction in which heat is absorbed and the temperature of the surroundings
falls
17. __________________ a quantity whose value is
determined only by the state of the system
18. __________________ the
enthalpy change for the hypotheorical formation of 1 mol of a substance in the
stanbdard state from the most stable forms of it constituent elements in their
standard states.
19. _____________________Heat flows into or out of a
thermodynamic system so that there is no temperature chane in the system.
20. _____________________No heat may flow into or out of a thermodynamic system. The system is
perfectly insulated from its surroundings