CHM 2046C Module Nine Homework Packet Name:___________
|
Module Nine: Chemical Kinetics - Chapter 12 |
Possible |
Actual |
|
|
5 |
|
|
|
|
B. Factors forming the rate Constant- |
5 |
|
|
|
C: Free Radical Mechanism of Halogenation of
Alkane (lecture) |
5 |
|
|
|
D. Determination of Rate Orders from Lab Data
|
10 |
|
|
|
5 |
|
|
|
|
F. Conct-Time Relationships Half Life 1st
Order |
5 |
|
|
|
10 |
|
|
|
|
H. Discussion Question-Chapter 12 and Kotz C15
study guide & Lecture |
5 |
|
|
|
I. Dependence of Reaction Rates on Temperature |
5 |
|
|
|
Module Nine Total: |
55 |
|
|
Part A: Law of Mass Action 5 points
In the reaction: A + B ------> C + D ; assuming the reaction is first order with respect to both the reactants A and B;
the rate expression is: Rate = k’[A][B]. The reverse reaction is not a factor in the initial concentration changes.
Demonstrate the Law of Mass Action in explaining why you
multiple the concentrations A times the concentration of B in the rate expression
( [A][B] ) .
What does this
product represent?
Is this product a
number greater or less than one?
Part B: Factors Forming Rate Constant: k’ 5 points
In the Rate expression in Part A: RateINT = dP/dT = k’[A][B];
Is the rate constant
k’ a number greater than one or less than one, why?
What does this number represent?
The rate constant k’ can be broken down into the product of four factors. What are the four factors and what does each factor represent? Are each of these greater than one of less than one?
1)
2)
3)
4)
Part C: Free Radical Mechanism Example 5 points
The free radical mechanism for the halogenation of alkane has been established as a chain reaction. Demonstrate this mechanism by showing all four steps in this mechanism using Methane and Bromine in Ultraviolet Light
CH4 + Br2 + uv light à CH3Br + HBr
a.
Chain initiation
step:
b.
Chain Propogation
Steps
c.
Chain Termination
Steps:
d. What evidences did
the chemist record which lead to this suggested mechanism?
Part D: Rate Laws from Experimental Data: Initial Rates Method 10 points
1) In the reaction: 2 NO (g) + Cl 2 (g) à 2 NOCl (g)
Was studied at -10 oC. The following results were obtained:
[NO] (mol/L) [Cl 2] (mol/L) Initial Rate (Mol/L min)
0.10 0.10 0.18
0.10 0.20 0.35
0.20
0.20 1.45
a. What is the Rate Law?
b. What is the rate
order of each reactant?
c. What is the
overall rate order of the reaction?
d. What is the value
of the rate constant k?
e. What are the units of the rate constant?
Part DContinued: Rate Laws from Experimental Data: Initial Rates Method
In the reaction: CO (g)
+ NO 2 (g) à CO2 (g) + NO
(g)
The following results were obtained:
Experiment [CO] (mol/L) [NO 2] (mol/L) Initial Rate (Mol/L hr)
#1 5.0 x 10-4 0.36 x 10-4 3.4 x 10-8
#2 5.0 x 10-4 0.18 x 10-4 1.7 x 10-8
#3 1.0 x 10-3 0.36 x 10-4 6.8 x 10-8
#4 1.5 x 10-3 0.72 x 10-4 ?
a. What is the Rate Law for this reaction?
b. What is the rate order of each reactant?
c. What is the overall rate order of the reaction?
d. What is the value of the rate constant k for this reaction?
e. What are the units of the rate constant?
f. What is the initial rate for the reaction in experiment #4?
Part E: 1st, 2nd, Zero Order Concentration-Time Relationships 5 pts
Given the following integrated expressions:
Zero order: [R]t – [R]0 = kt
1st order: ln ([R]t / [R]0 )= -kt
2nd order: 1/[R]t – 1/[R]0 = kt
(18) The decomposition of N2O5 in CCl4 is a first-order reaction. If 2.56 mg of N2O5 is present initially, and 2.50 mg is present after 4.26 minutes at 55oC,
what is the value of the Rate constant k?
(21) Ammonium Cyanate, NH4NCO, rearranges in water to give urea:
NH4NCO (aq) à (NH2)2CO (aq)
The rate expression for this process is Rate = k[NH4NCO]2 where k=0.0113 L/mol min.
If the original concentration of the NH4NCO in solution is 0.229 mol/L, how long will take
for the concentration to decrease to 0.180 mol/L?
Part F: Concentration-Time
Relationships Half Life 1st
Order 5 pts
Use the following t½ = 0.693/k
to solve:
Sucrose, C12H22O11,
decomposes to fructose and glucose in acid solution with the rate law:
Rate = k[C12H22O11] k = 0.208 h-1 at 25 oC
What amount of time is
required for 75.0% of the initial concentration of sucrose to decompose?
87.5%?
93.75% ?
Part G: Reaction Mechanisms 20 points
1)The mechanism for the decomposition of hydrogen peroxide is:
H2O2 à 2 OH
H2O2 + OH à H2O + HO2
HO2 + OH à H2O + O2
If the order of the reaction is first order with respect to hydrogen peroxide and overall the reaction is first order; which step(s) above is(are) the rate determining steps?
Which species are reaction intermediates?
2)The rate law for the reaction:
BrO3 1- (aq) + 3 SO3 2 - (aq) à Br 1- (aq) + 3 SO4 2 - (aq)
is: Rate = k’[BrO3 1-] [ SO3 2 -] [ H 1+]
The first step in the mechanism is:
SO3 2 - (aq) + H 1+ (aq) -----------> H SO3 1 - (aq) FAST
The second step is rate determining. Write a possible second step for the mechanism.
3) For the reaction:
2 H2
(g) + 2 NO (g) à N2
(g) + 2 H2O (g)
the observed rate law is:
Rate = k[NO]2[H2]
The mechanisms show below have been proposed to explain the kinetics of the
reaction:
2 H2
(g) + 2 NO (g) à N2
(g) + 2 H2O (g)
Mechanism I:
2 H2
(g) + 2 NO (g) à N2
(g) + 2 H2O (g)
Mechanism II:
H2
(g) + NO (g) à H2O
(g) +
N (g) Slow
N (g)
+ NO (g) à N2
(g) + O (g)
Fast
H2 (g) + O (g) à H2O
(g) Fast
Mechanism III:
H2 (g) + 2 NO (g) à H2O
(g) +
N2O (g) Slow
N2O(g) + H2 (g) à N2
(g) + H2O (g) Fast
Which of the above is(are) the acceptable mechanisms? Explain.
List the reaction
intermediates:
4) A proposed mechanism for a reaction is
C4H9Br à C4H91+ + Br 1- slow
C4H91+ + H2O à C4H9OH21+ fast
C4H9OH21+ + H2O à C4H9OH + H3O
1+ fast
Write the rate law expected for the mechanism:
What is the overall balanced equations for the reaction?
What are the intermediates in the proposed mechanism?
------------------------------------------------------------------------------------------------------
5) The mechanism for the reaction of nitrogen dioxide with carbon monoxide to form nitric oxide and carbon dioxide is thought to be
NO2
+ NO2 à NO3 +
NO slow
NO3
+ CO à NO2 + CO2 fast
Write the rate expected for this mechanism.
What is the overall balanced equation for the reaction.
Part H: Discussion Question-Chapter 12 5 points
Work the four circled questions. If none are circled ask you instructor:
1. Summarize the ways in which the rate of a chemical
reaction can be changed.
2. What is a reaction intermediate?
3. What does a catalyst actually do? What is the difference between a homogeneous
catalyst and a heterogeneous catalyst?
4. List the three requirements for a successful
collision between molecules.
5. Define the rate-determining step of a reaction
mechanism.
6. Describe what the activation energy is. Show on a
reaction diagram using energy vs reaction progress
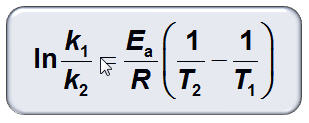
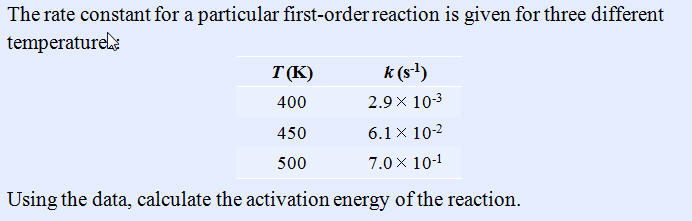
Part I: Dependence of Reaction Rates on Temperature 10 points
Use the following Equation: