CHM 1032C Tentative Grading Outline Fall 2015
Chapter 5 Classification & Balancing
Chemical Reactions
E._____ (02)
Writing
Reactions/ Symbols-Page 7 & Section
5.1 Answers
E1.____ (02) Classifying
Chemical Reactions- Lecture & Section 5.3 Answers
F._____ (04) Balancing Chemical
Equations -Sections 5.2 Answers
ef
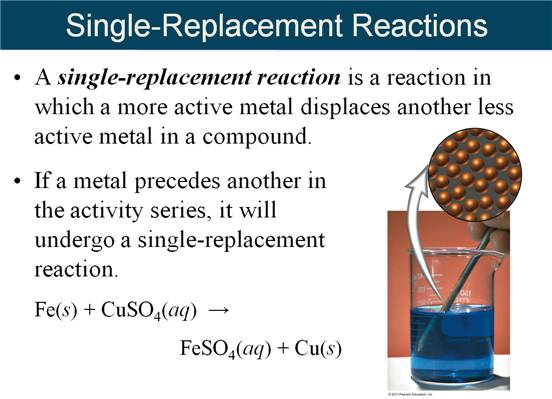
G._____(02) Predicting Single
Replacement Products Lecture Answers
H._____( 02) Predict Double Replacement Sections
5.4 Answers
h
H1____ (02) Neutralization/Gas Forming Reactions Section 5.5 & Lecture
Answers
W. _____(06) Rewrite Equations Ionically –Section 5.8 Answers
R.
_____(06) Redox
Equations-Sections-Section 5.6-5.7 Answers
______(26) Total
= ______%
McMurry GOB Chapter 5 Table Contents
5. Classification and Balancing of Chemical Reactions
5.1 Chemical Equations M-5E
5.2 Balancing Chemical Equations M-5F
5.3 Classes of Chemical Reactions M-5E1
5.4 Precipitation Reactions and Solubility Guidelines M-5H
5.5 Acids, Bases, and Neutralization Reactions M-5H1
5.6 Redox Reactions M-8H
5.7 Recognizing Redox Reactions
5.8 Net Ionic Equations M-8G
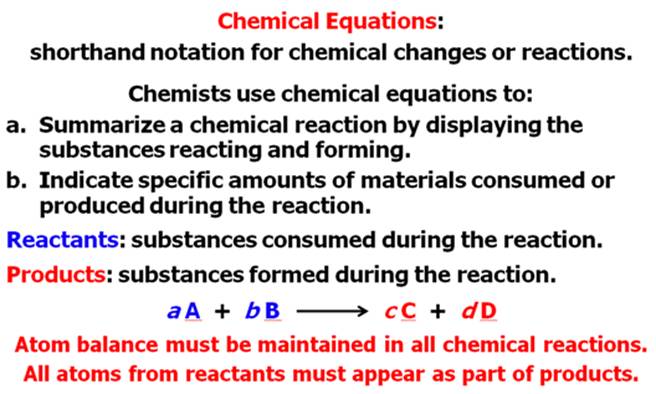
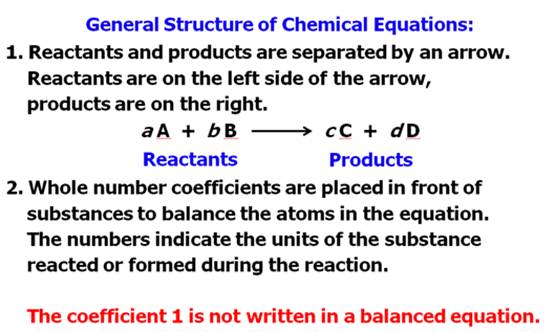
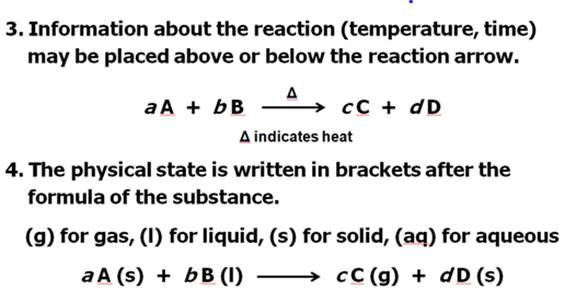
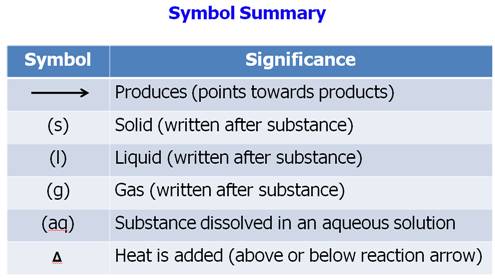
From Your Hein Textbook and Power Point for
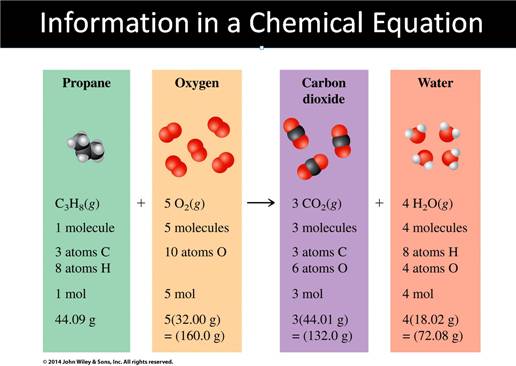
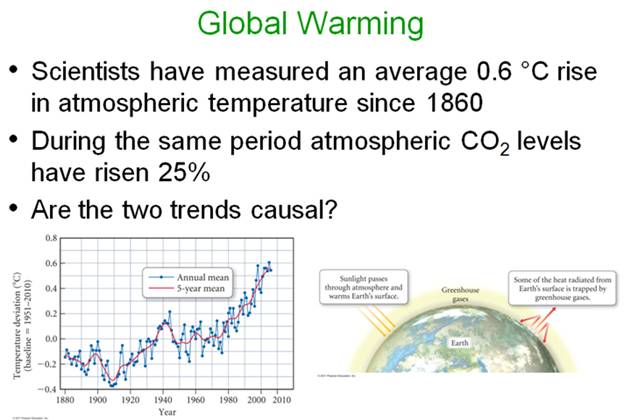
Chapter 8, what is a chemical equation?
Evidence for
Chemical Reactions
There are four observations that indicate a
chemical reaction is taking place:
1.
A gas is produced.
Gas may be observed in
many ways in a reaction from light fizzing to heavy bubbling.
2.
An
insoluble solid is produced
in a solution.
a. A substance
dissolves in water to give an aqueous solution.
b. If we add
two aqueous solutions together, we may observe the production of a solid
substance.
c. The
insoluble solid formed is called a precipitate
3. A permanent color change is observed.
a. Many
chemical reactions involve a permanent color change.
b. A change in
color indicates that a new substance has
been formed
4. An energy change is observed
a. A reaction
that releases heat is an exothermic reaction.
b. A reaction
that absorbs heat is an endothermic reaction.
c. Examples of
a heat energy change in a chemical reaction are heat and light being given off.
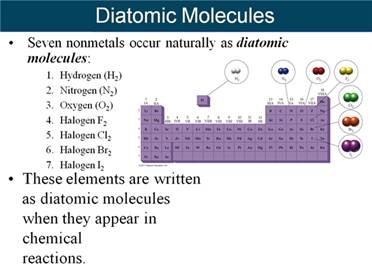
From the Corwin Textbook, the Chemical symbols are explained:

Chapter 5 Homework Packet
Chapter
5-Part E Basic Stoichiometry
Definitions 2 points
Fill in the
following with the symbols used in chemical equations which has the stated
translation or meaning(s) (Section 7.2 Table 7.1 page 191 Corwin 7th ) (Hein Section 8,1 page 144)::
_________1.
Produces, yields, gives
_________2.
Reacts with, added to, plus
_________ and _________ 3. Solid substance or precipitate forms
_________and _________4. Gaseous substance formed
_________5.
Liquid Substance
________5a. Water or aqueous solution
_________6. Reversible Reaction
_________7.
No Reaction
8. Show the
symbol for heat:__________
9. How
would you show a catalyst in a chemical reaction where A plus B forms products
D and E, but is catalyzed by substance C
A + B à D + E
10. Define
Catalyst (See Section 7.6 page 193)
Chapter 5 Homework Packet
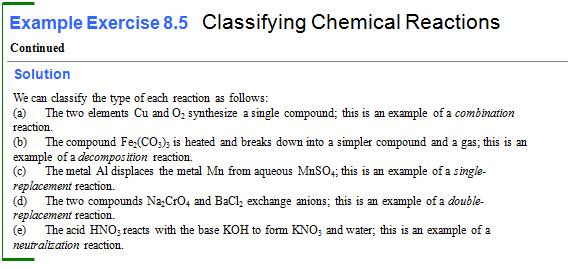
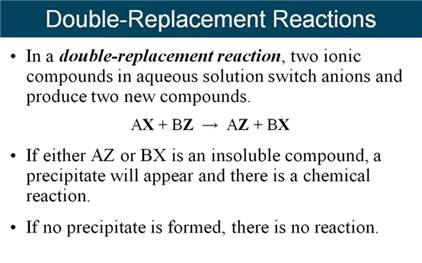
Although McMurry does not define the following five types of reactions: Combination, Decomposition, Single Replacement, Double Replacement, and Neutralization reactions are introduced. The table 8.3 from another text is a summary.
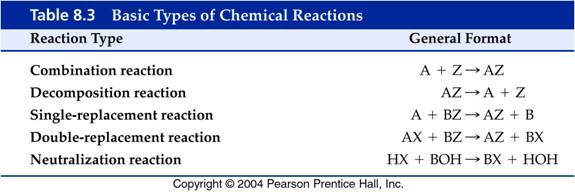
From another book:
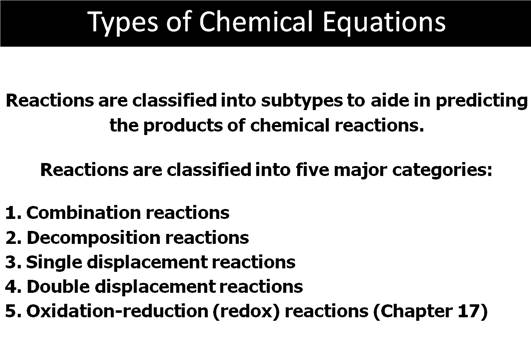
From lecture, I will tell you a chemical change via a chemical reaction
is either a NON-REDOX or a REDOX change. Combination, Decomposition, Single
Replacement and Oxidation-Reduction (more complicated) are REDOX changes. Only
Double Replacement, Double Displacement or sometimes called Metatarsus
reactions are NON-REDOX and I like to call them Ion-Exchange Reactions,.
Our text
discusses precipitation reactions in section 5.4 , but
leave off:
Gas Forming Reactions.
When
predicting the products of a double replacement reaction, sometimes one of the
products instantly decomposes.
If H2CO3
is a predicted product in ion exchange, it is written as
CO2 and H2O.
For example:
Na2CO3
(aq) + HCl (aq) à
[H2CO3](aq) + NaCl (aq)
Should
be written:
Na2CO3
(aq) + HCl (aq) à
CO2(g) + H2O (l) + NaCl (aq)
Two
other products which are shown differently:
[NH4OH] à NH3 + H2O
[H2SO3 ] à SO2 + H2O
In Section
12.8, there is a another type of chemical reaction:
Combustion – a substance burns in the
presence of oxygen. Combustion of a compound that contains C and H (or C, H,
and O) produces carbon dioxide gas and water.
CH2O(l) + O2(g)
→ CO2(g) + H2O(l)
The general type of combustion problem looks
like this:
CxHy [(l) or (g)] + O2 (g) à CO2 (g) + H2O
(g)
Or
CxHyOz[(s) or (l) or
(g)] +
O2 (g) à CO2 (g) + H2O
(g)
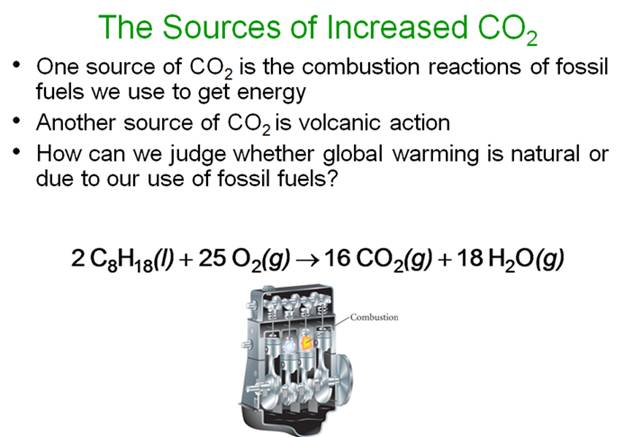
The
chemical reaction for the combustion of gasoline:
C8H18
(l) + O2 (g) à CO2
(g) +
H2O (g)
Octane oxygen gas carbon dioxide water
Module 5 E1: Classification of Chemical Reactions 2 Points
(Sections 7.4)
Classify Each of the
following (unbalanced) chemical reactions as:
- Combination
(or synthesis)
- Decomposition
(or Anaylsis)
- Single
Replacement
- Double
Replacement (Precipitation)
- Double
Replacement (Neutralization)
- Double
Replacement (Gas Forming)
- Combustion
of a hydrocarbon
______1. Fe
+ FeCl3 à FeCl2
____2. HCl + Mg(OH)2 à MgCl2 +
HOH
_____3. Mg +
HNO3 à Mg(NO3)2 +
H2
_____4. H2 +
N2 à NH3
____5.
NaHCO3 + HCl à NaCl + CO2 + H2O
____6.
Ca(NO3)2 + K3PO4 à Ca3(PO4)2 + KNO3
____7.
KClO3 à KCl +
O2
____8.
Na + H2O à NaOH + H2
Writing
Chemical Reactions (Section 5.1-5.6)
9.
Write a chemical equation for solid cadmium hydrogen carbonate decomposing to
yield solid cadmium carbonate, water, and carbon dioxide gas:
10.
Write a chemical equation for the reaction of aqueous solutions of potassium
chromate and calcium sulfate to give the precipitate calcium chromate and
aqueous potassium sulfate.
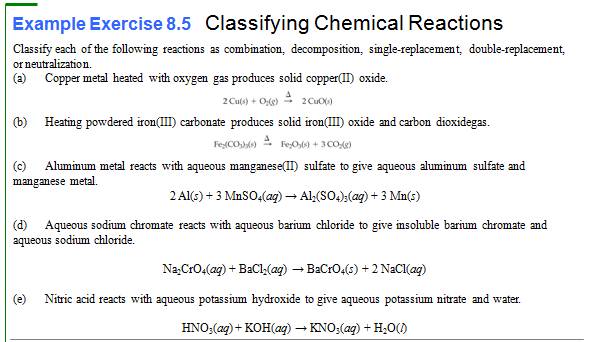

Writing
Chemical Reactions
11.
Write a chemical equation or solid sodium hydrogen carbonate decomposing to
yield solid cadmium carbonate, water, and carbon dioxide gas:
12.
Write a chemical equation for the reaction of aqueous solutions of potassium
chromate and lead(II) nitrate to give the precipitate
lead(II) chromate and aqueous potassium nitrate.
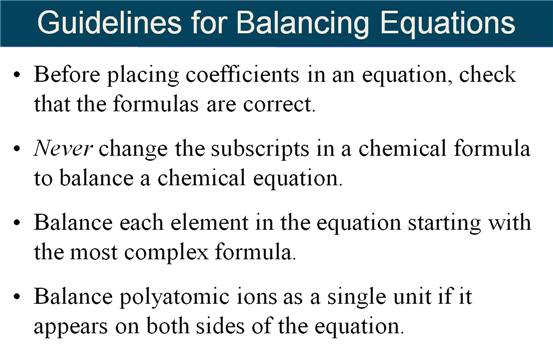
Rules and Suggestions
for Balancing Equations
1)
The same # and
type of atom must be present on each side of the equation.
2)
Balancing is
accomplished by adding coefficients. NEVER change the subscripts.
3)
Coefficients must
be in the smallest whole # ratio.
4)
Balancing is done
by trial and error.
5)
Usually Balance
H’s and O’s last or an element that appears in more than one place of either
side of the reaction .
6) Balance polyatomic ions as one unit in Ion Exchange reactions.

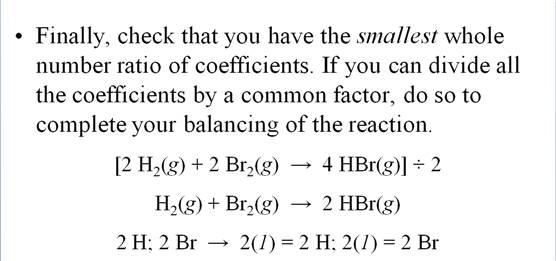
Chapter 5 Homework Packet
Example of balancing an Equation:
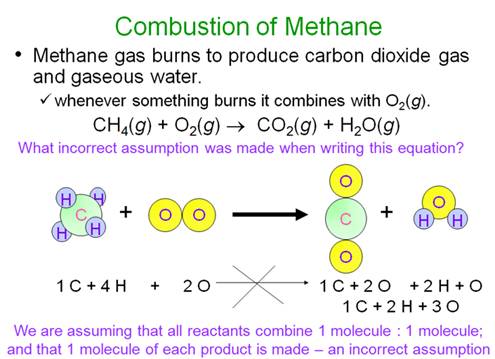
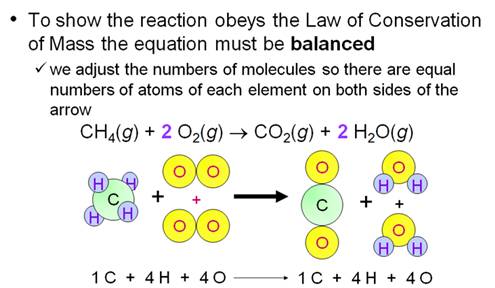
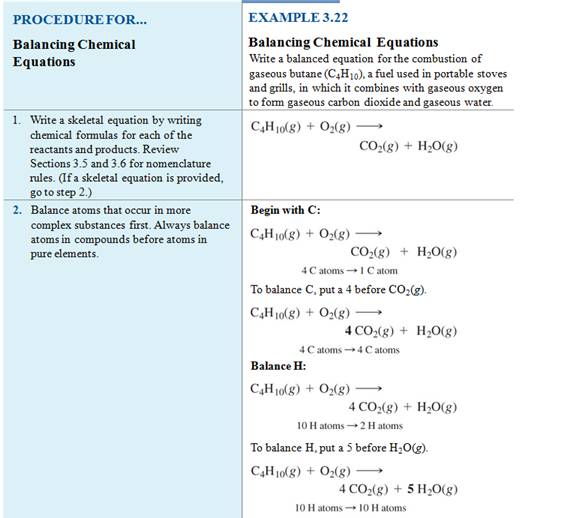
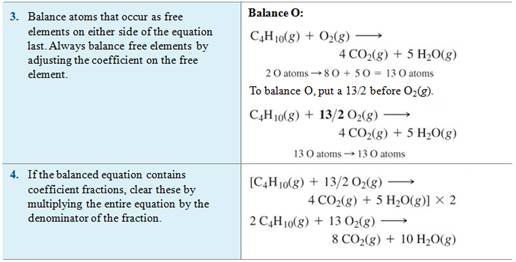
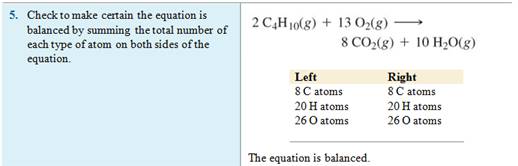
Chapter
5-Part F Balancing Chemical Equations
4 points
Balance the
following chemical equations (write the chemical formulas in #10 then balance):
1. Fe
+ FeCl3 à FeCl2
2. Al +
O2 à Al2O3
3. Na2CO3 +
C + N2 à NaCN +
CO
4. FeS +
O2 à Fe2O3 +
SO2
5. IBr +
NH3 à NI3 + NH4Br
6. Cl2 +
HOH à HCl + HClO
7. AgNO3 à AgNO2 +
O2
8. HClO4 +
P4O10 à H3PO4 +
Cl2O7
9. HCl + Mg(OH)2 à MgCl2 +
HOH
10.
Sodium hydroxide + Hydrochloric
acid à sodium chloride +
water
You
may check your work using the online chemical equation balancer at:
http://people.emich.edu/bramsay1/ccc-release/chem.html
Chapter 5 Homework Packet

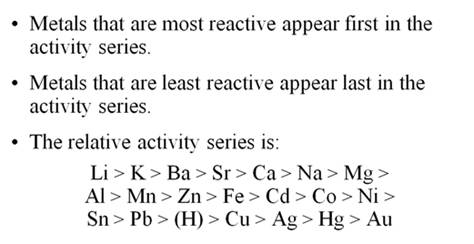
Chapter 5 Homework Packet

Chapter 5 Homework Packet
Chapter 5 Homework Packet
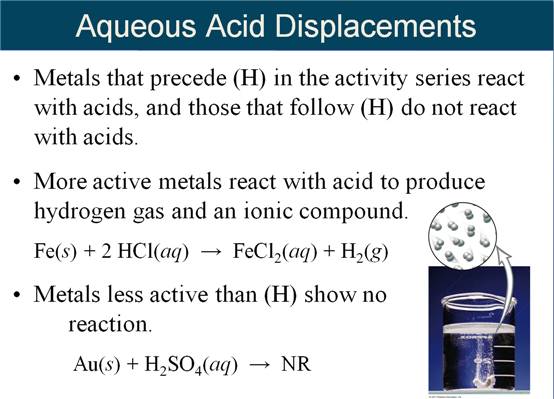
If you were to (H2O) in the activity
series like an acid is shown as (H), where would you put it? Show below:
Given
the following Activity Series:
Li > K
> Ba > Sr > Ca > Na >
Mg > Al >
Mn > Zn > Fe > Cd > Co > Ni >
Sn >
Pb > (H) > Cu >
Ag > Hg > Au
The rule to follow is a single replacement reaction takes place only if the metal or (H) is more active than the metal or (H) it is replacing. Li will react with everything, while Hg will replace only gold. And poor gold does not react with any of the cations of metals. Therefore gold is found pure in nature, while the very active metals such as potassium and sodium are never found pure in nature, but are found as minerals (ionic compounds).
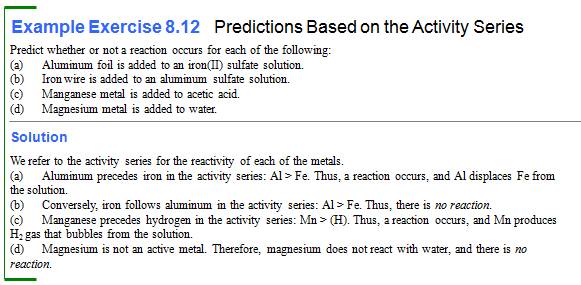
Will Mg metal react with Nitric Acid?
Yes
(Mg has a great
reactivity then [H] in the series)
Mg (s) + 2 HNO3 (aq) → Mg(NO3)2 (aq) + H2 (g)
Will Copper react with Nitric Acid? no
(Cu is below [H] in
the activity series)
Cu (s) + HNO3 (aq) → no reaction
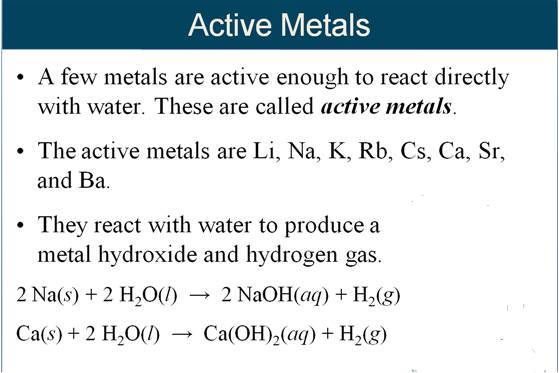
Given the following Active Metals:
Li > K > Ba > Sr > Ca > Na> (H2O)
The six very active metals are so reactive they will replace one of the two hydrogens in water and form alkaline hydroxides as products. Hydrogen gas will bubble out of the solution. See some of the above movies for demonstrations.
Will Sodium react with water? yes
(Na is one of the six
active metals above)
2 Na(s) + 2 HOH (l) → 2 NaOH (aq) + H2 (g)
Chapter 5 Homework Packet
Part
G Single Replacement Reactions 2 points
Given
the following Activity Series:
Li
> K > Ba > Sr >
Ca > Na > Mg > Al > Mn > Zn > Fe
> Cd > Co > Ni >
Sn > Pb > (H) > Cu
> Ag > Hg > Au
Given
the following Active Metals:
Li
> K > Ba > Sr >
Ca > Na
Complete
the products of the following reactions, then balance the equation (If no
reaction write NR):
1. Cu (s) + Al(NO3)3 (aq) à
2. Al (s) +
Cu(NO3)2 (aq) à
3. Au (s)
+ H2SO4 (aq) à
4. Ca (s) +
H2O (l) à
5.
Mn (s) + H2O
(l) à
Chapter 5 Homework Packet
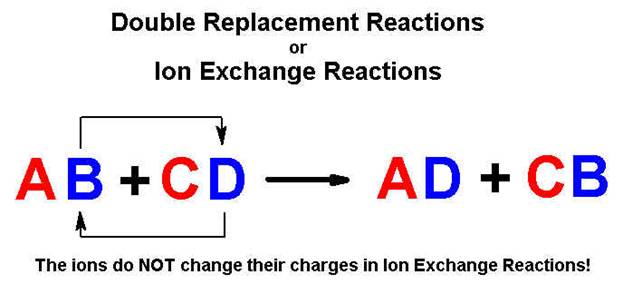
Except in neutralization and gas forming reactions.
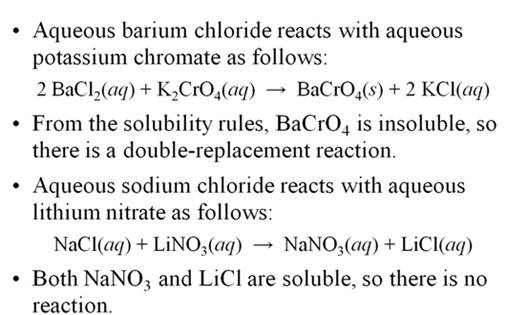
Chapter
5 Part H Double Replacement
Reactions 2
points
Given the following Solubility Rules for Ionic
Compounds:
Compounds containing the following ions are
generally soluble in water:
1. Alkali metal ions and ammonium ions, Li+ , Na+ , K+ , NH4+
2. Acetate ion, C2H3O2-
3. Nitrate ion, NO3-
4. Halide ions (X), Cl-
, Br- , I- (AgX, Hg2X2 , and PbX2 are insoluble exceptions)
5. Sulfate ion, SO4 2-
(SrSO4, BaSO4
, and PbSO4 are
insoluble exceptions)
Compounds containing the following ions are
generally insoluble in water:
6. Carbonate
ion,CO32- (see rule 1
exceptions which are soluble)
7. Chromate
ion CrO42- (see rule 1 exceptions which are soluble)
8. Phosphate ion PO43-
(see rule 1 exceptions which are soluble)
9. Sulfide
ion, S2- (CaS, SrS, BaS, and rule 1 exceptions
are soluble in water)
10. Hydroxide ion, OH- [ Ca(OH)2
, Sr(OH)2 , Ba(OH)2
, and rule 1 exceptions are soluble)
Complete and balance the following reactions using
the above solubility table (write no reaction or NR if both products are
soluble or a covalent compounds is not formed)
1. AlCl3
(aq) + K2CO3 (aq) à
2. NiSO4
(aq) +
Li3PO4 (aq) à
3. NaCl (aq) +
AgNO3 (aq) à
4. H2SO4
(aq) +
NaOH (aq) à
5. H3PO4
(aq) +
Ba(OH)2 (aq) à
Video:
http://www.brightstorm.com/science/chemistry/chemical-reactions/double-replacement-reactions/
http://www.youtube.com/watch?v=7hVKb4ROjZw
http://www.youtube.com/watch?v=_oixjNeKtxs
http://www.youtube.com/watch?v=IMfNi_C2DTg
http://www.youtube.com/watch?v=2tIutF6-wn4
Chapter 5 Homework Packet
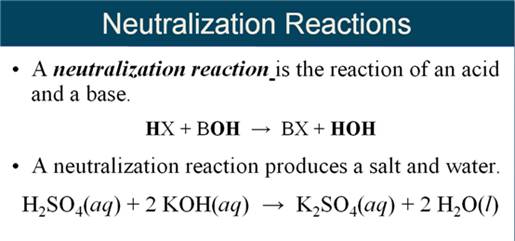
Chapter
5 Part H1 Double Replacement Reaction:
Neutralization/Gas Forming
Reactions 2 points
Complete and balance the following precipitation
reactions using the above solubility table
(write no reaction if both
products are soluble or a covalent compounds is not formed)
1. Mg(OH)2 (s)
+ H2SO4 (aq) à
2. H3PO4
(aq) +
KOH (aq)
à
3. NH4NO3
(aq) +
Ba(OH)2 (aq) à
4. HBr (aq) +
Pb(CO3)2 (aq) à
5. LiOH (aq) + H3PO4 (aq) à
6. Na2CO3
(aq) +
HCl (aq) à
Video:
http://www.youtube.com/watch?v=gtcE8TosEq4
Note for Chapter-5H1:
Neutralization ion Exchange Reaction:
1. When an acid
reacts with a base, salt plus water are the products
Gas Forming Ion Exchange Reactions:
2. When either H2SO3;
H2CO3 or NH4OH is formed as a product it
immediately
decomposes thus demonstrating a gas forming
reaction.
3. Most books do not
show either H2CO3 or NH4OH as products, just
the
decomposed products of the
gases and water in the answer.
If H2CO3
is a predicted product in ion exchange, it is written as
CO2 and H2O.
For example:
Na2CO3
(aq) + HCl (aq) à
[H2CO3](aq) + NaCl (aq)
Should
be written:
Na2CO3
(aq) + HCl (aq) à
CO2(g) + H2O (l) + NaCl (aq)
Two
other products which are shown differently:
[NH4OH] à NH3 + H2O
[H2SO3 ] à SO2 + H2O
Chapter 5 Homework Packet
Chapter
5: Part W Rewriting Equations Ionically 2 points Plus additional homework
Rewrite the
following (unbalanced) equations ionically, cancel spectator
ions and then balance the net ionic reactions.
Show as ions: soluble salts and
strong acids and strong bases;
leave as molecules/formula units insoluble salts, weak acids, covalent molecules.
Strong acids are: Perchloric
Acid; Hydrochloric Acid; Nitric Acid; Sulfuric Acid; Hydrobromic
Acid; Hydroiodic Acid.
Strong
bases are Sodium hydroxide, Potassium hydroxide, Calcium hydroxide, Barium
hydroxide and Strontium hydroxide
1.
KOH (aq) + HNO3(aq) à KNO3(aq) + HOH(l)
2. CuSO4 (aq) + Na2CO3 (aq) à CuCO3 (s)
+
Na2SO4 (aq)
3. NaOH (aq) +
NH4NO3 (aq) à NaNO3 (aq) + NH3 (g) +
HOH (l)
4. BaBr2 (aq) + ZnSO4 (aq) à BaSO4 (s) +
ZnBr2 (aq)
5. Cr(OH)2
(s) +
HCl (aq) à
Video:
http://www.brightstorm.com/science/chemistry/chemical-reactions/net-ionic-equation/
Chapter 5 Homework Packet
Chapter
5 Part R: Redox
Equations 2 points plus additional
homework
Balance the
following redox equations written in net ionic form:
Acid Media: (1 point)
1. C2O4
2- (aq) +
MnO4 1- (aq) + H 1+ (aq)
→ Mn 2+ (aq) + CO2
(g) +
HOH (l)
half equation:
half equation:
Basic Media (1 point)
2. Bi2O3
(s) + OH 1- (aq) + OCl 1-
(aq) → BiO3
1- (aq) + Cl 1-
(aq) + HOH (l)
half equation:
half equation:
Separate Handouts and Additional Homework for Parts R & W will be distributed