Path
4: Chapter
3:
Elements of Chemistry Study Pack
Part
A: Chemical
and Physical Properties Answers Section 3.1
Part A1: Chemical
and Physical Change Answers Section
3.1
Part B: Elements and
Symbols Answers Section
3.2
Part C: Element
Classification Answer Section
3.3
Part D: Compounds and
Chemical Formulas Answers Section 3.4
Part E: Binary Molecular
Compounds Answers Section
3.5
Part E1: Polyatomic
Ions Answers Section
3.5
Part E2 Ternary
Ionic Compounds Answers Section
3.5
Part
E3: Binary/Ternary
Acids Answers See Study Guide/not in Chapter
Part F: Binary Ionic
Compounds Answers Section
3.5
Part G: Matter Chart Answers Section 3.7
Part P: Periodic
Properties Answer Section 3.3
Part A: Chemical/Physical/Nuclear
Properties (Section 3.1)

Chapter 3 Part A: Chemical & Physical Properties
1. Define:
Physical Property:
2. Chemical
Property:
3. Classify each
of the following as a chemical or physical property:
a.
Color ________________
b.
Odor _________________
c.
Reaction with water:
____________________
d.
Solubility in water: ____________________
e.
Melting point: ____________________
f.
Boiling point: ____________________
g.
Sublimation Point: ____________________
h.
Reaction with oxygen ____________________
i.
Density:
____________________
j.
Solid state: ___________________
k.
Reaction producing a Gas: _____________________
l.
Conductor of electricity: _____________________
m.
Water is insoluble in gasoline:
_____________________
n.
Good conductor of heat: _____________________
o.
Two chemical when mixed
gives of heat: ___________________
p.
Appearance at Room Temperature: ___________________
q.
An element turns black when heated ___________________
r.
Silver tarnishes in Air ____________________
s.
An element is radioactive: ____________________
Part A1: Chemical/Physical/Nuclear
Change (3.1)


Chapter 3 Part A1: Chemical & Physical Change
State whether each
of the following is a physical change, a chemical change, or a nuclear
change:
__________________1.
Electricity decomposes water.
__________________
2. Methanol dissolves in gasoline
__________________
3. Dry ice pellets disappear
__________________
4. Iron oxidizes to rust
__________________
5. Bromine vaporizes into a reddish-brown gas
__________________
6. Uranium-235 splits into two small elements when bombarded
with
neutrons in an atomic bomb.
___________________7.
Copper conducts heat
___________________8.
Baking soda fizzes in vinegar
___________________9.
Grinding sugar crystals into a powder
__________________10.
Sodium reacts with chlorine gas
__________________11.
Adding air to a tire
__________________12.
Slicing an orange into wedges
__________________13.
Hydrogen atoms fuse into helium atoms in a hydrogen bomb
__________________14.”
Dry ice”(Solid Carbon dioxide) vaporizes into a gas at
room
temperature
and sea level pressure
__________________15. Natural Gas burs with a blue flame
Part
B: Elements and Symbols (Section 3.2)


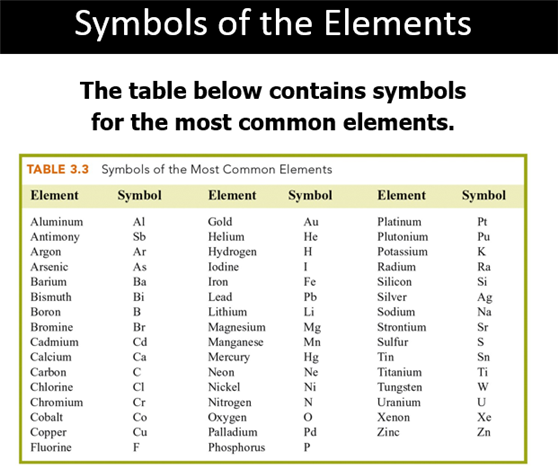
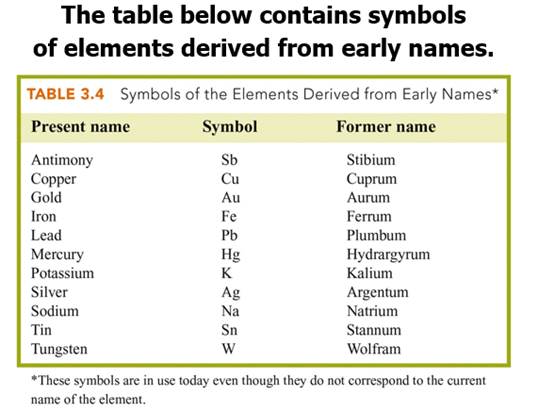

Chapter
3 Part B: Elements/Symbols
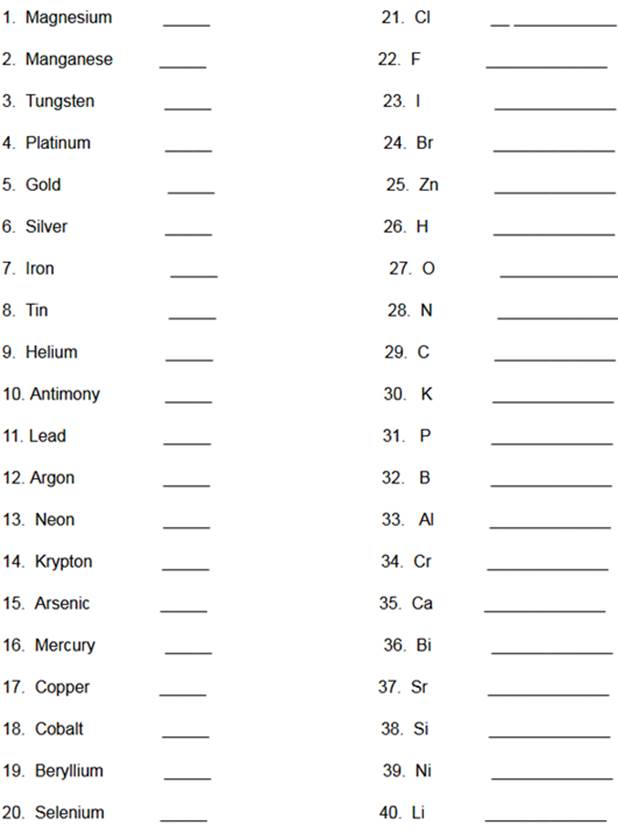
Element Identification Homework Click
on Element for Answer
Identify each of the following elements chalk board representation:


1. Element: _______________Symbol:_____ 2.Element: _______________Symbol:_____
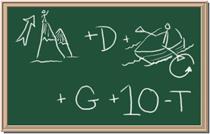

3. Element: _______________Symbol:_____ 4.Element: _______________Symbol:_____

5. Element: _______________Symbol:_____ 6.Element: _______________Symbol:_____
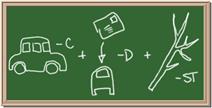
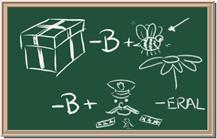
7. Element: _______________Symbol:_____ 8.Element: _______________Symbol:_____

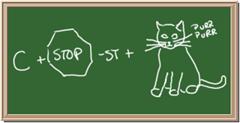
9. Element: _______________Symbol:_____ 10.Element: _______________Symbol:_____
Part C: Element Classification Section 3.3
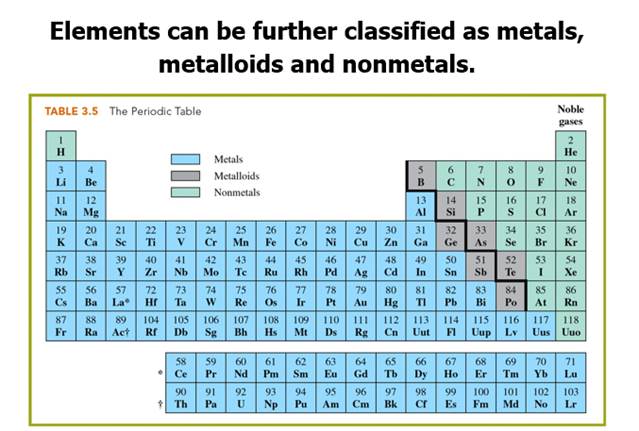
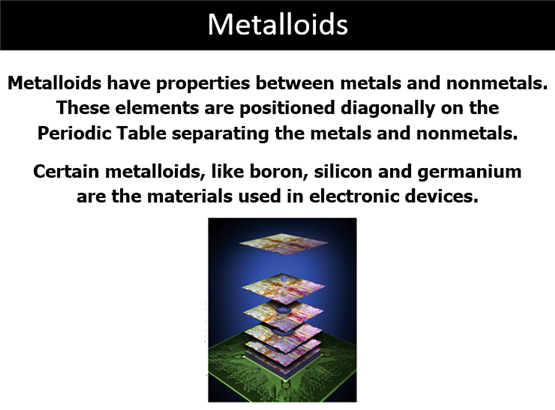
Part C: Element Classification Sample Test
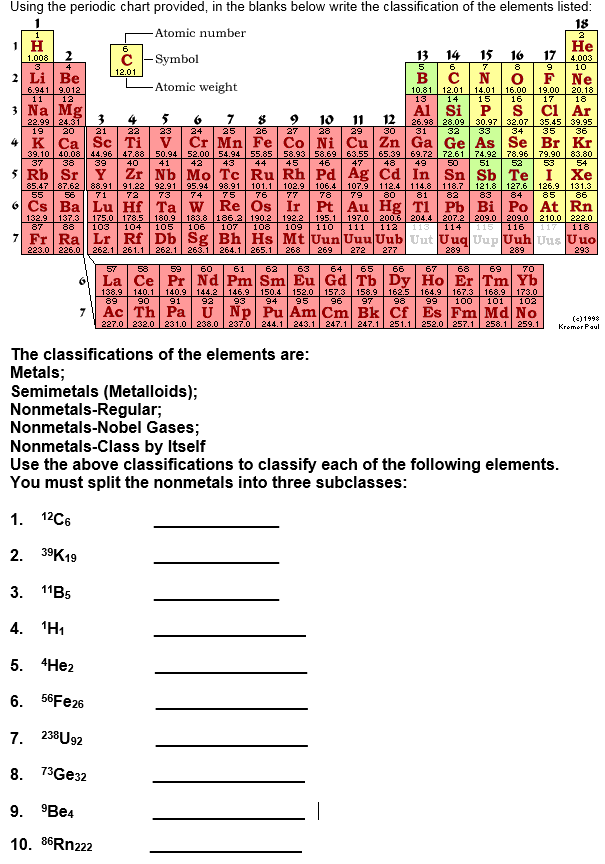
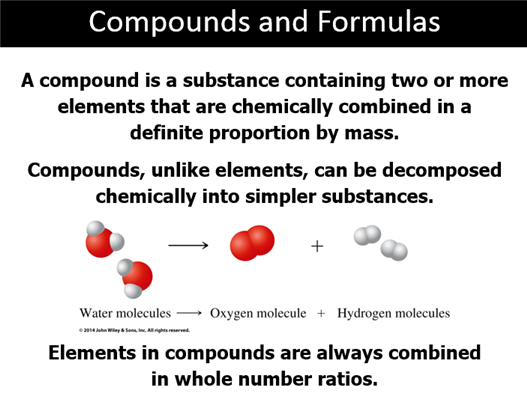
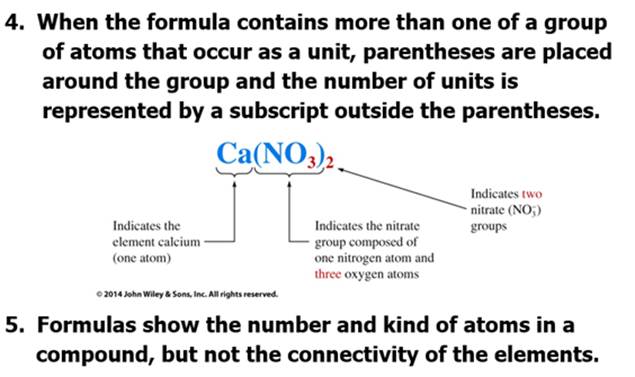
Part D: Compounds and Chemical Formulas (Sect 3.4)
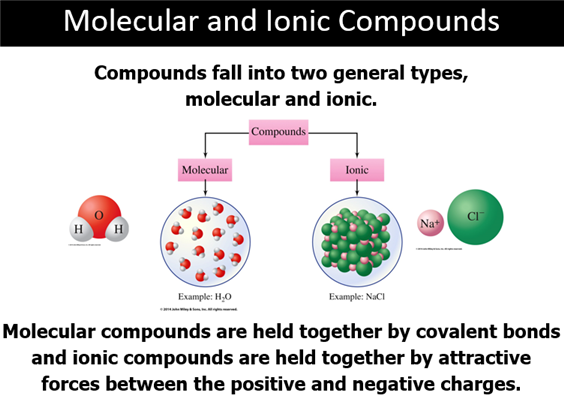
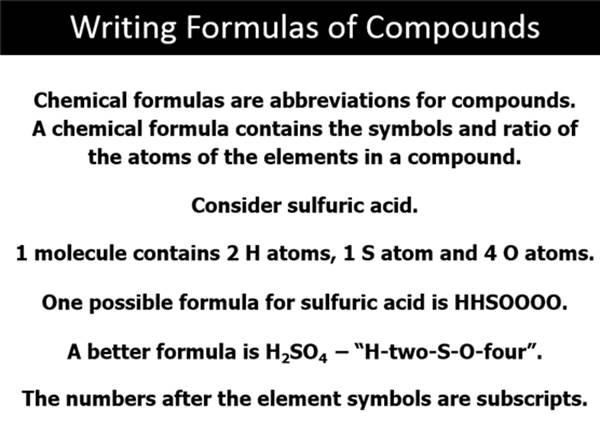
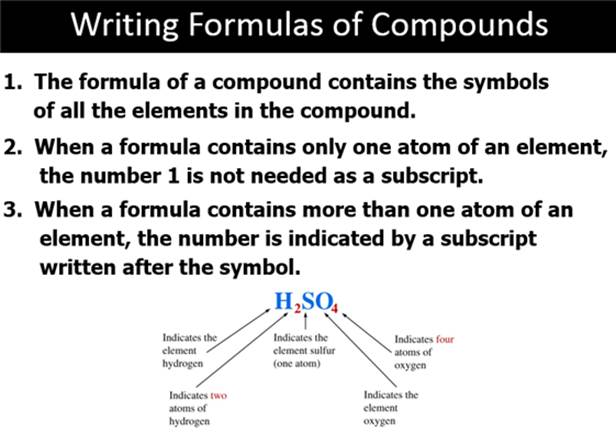



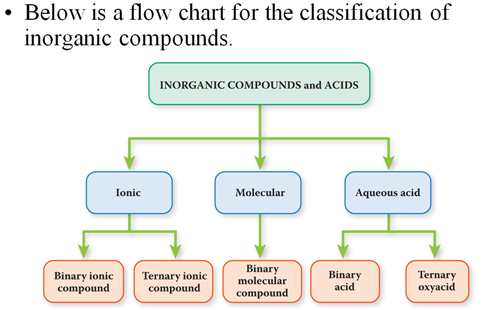
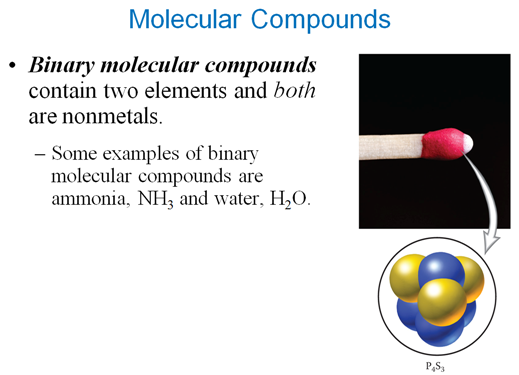
Chapter 3 Part E: Binary Molecular Compounds Section 3.5
The required Online Binary Covalent Molecular Homework
The
web site is:
C: Binary Molecular Names:
http://www.northcampus.net/Nomenclature/Molecules/25BinaryCovalent.html
C1:
Binary Molecular Formulas:
http://www.northcampus.net/Nomenclature/MoleculeFormula/25BinaryMolecularFormula.html
Here is a brief tutorial for Part E:
PART E: BINARY COVALENT COMPOUNDS
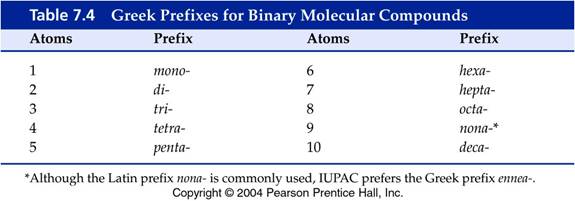
Both elements are nonmetals attached by covalent bonds. These bonds may be single, double, or triple covalent. Due to the covalent bonding there are many ratios of the same two elements making many different compounds. For this reason, the chemist states how many atoms of each element is present in the chemical formula in the formal name of the compound.


The element that is shown first in the chemical formula is written first using the proper prefix to indicate how may atoms of that element is contained in the compound. If there is only one atom of that element it is often found without the prefix mono. If you leave the prefix off then it is understood that you mean mono.
The element which is written second in the chemical formula is written second in the chemical name, but in addition to the prefix indicating how many, the suffix of the element’s name is changed to -ide.
carbon becomes carbide chlorine becomes chloride
sulfur becomes sulfide oxygen becomes oxide
hydrogen becomes hydride nitrogen becomes nitride
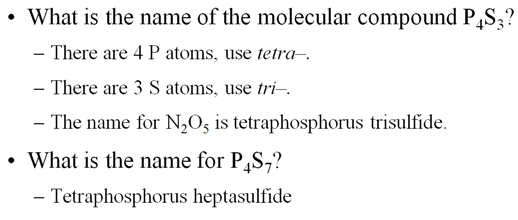
Therefore, the following formulas of binary compounds would be spoken:
CCl4 carbon tetrachloride
SO2 sulfur dioxide
CO2 carbon dioxide
N2O3 dinitrogen trioxide
BH3 boron
trihydride
We use common names for NH3, and H2O. What
would be their correct binary molecular names?
Methane, CH4, is
the organic name for CH4, what would its inorganic name be?
Chapter 3:
Part E Binary Molecular Compounds
Using a periodic chart write the names or formulas of the following
compounds depending on whether the formula or name is given:
Homework Packet Sample test: answer on grading outline
1. CO ____________________
2. SO3 _____________________
3. N2O5 _____________________
4. N2O7 _____________________
5. N2O
_____________________
6. Phosphorus
pentachloride _________
7. Boron trifluoride _________
8. Carbon dioxide _________
9. Sulfur Trioxide _________
10. Carbon Tetrachloride _________
Chapter 3: PART F:
BINARY (IONIC) COMPOUNDS
Section 3.5
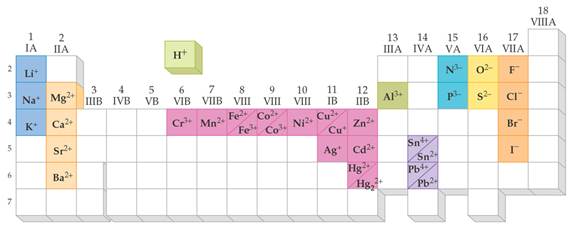
Most Common Ionic
Charges for Monatomic Ions
PART F:
BINARY (IONIC) COMPOUNDS
The element written first
in either the name or the formula is a metal. The element written second
is a nonmetal. Salts are metallic and nonmetallic ionic
compounds. There are no molecules of salts-just macro ionic
lattices. Name the metallic element.
If the metallic element has more than one ionic state, write a ROMAN NUMERAL after the element’s name (In Parenthesis) to indicate which charge state the metallic element is using to form the compound.
Drop the suffix off the nonmetal’s name and add -ide which indicates the salt is binary
(exceptions: cyanide & hydroxide which are polyatomic ions).
No prefixes are used to indicate how many atoms are present in the
formula.
Examples:
NaCl
Sodium Chloride (table salt)
Al2O3 Aluminum
oxide
FeS Iron(II) sulfide (Note: No
space between the metal and the parenthesis)
Fe2O3
Iron(III) oxide (rust)
To write the formula from the name of the salt use the following procedure:
(a) Write the symbols
(or formulas for radicals) of the ions represented
For Example:
Calcium nitride
(a)
Ca N
(b) Use the periodic chart to write the ion charge of each element (or polyatomic ion) as superscripts:
Ca+2
N-3
(c ) Find the L.C.M. (Least common multiple) of the positive and negative charge.
The LCM is the smallest number that both charges will decide into evenly. The LCM is the total electrons transferred. Therefore, it represents the total positive charge created by the metallic ions and the total negative charge created by the nonmetallic ions. This may be proved by drawing the dot structure of the compound showing all electrons transferred.
The LCM of +2
and -3 is 6, therefore 6 e-1
are transferred creating a total positive charge of +6, and the total negative charge
of -6
--> 6e-1-->
Ca+2
N-3
(d (d) Divide the LCM by the
positive charge, this dividend will represent the subscript behind the
metallic ion in the formula.
+6 divided by +2 = 3; therefore half of the formula is: Ca3Nx
(e) Divide the LCM by the negative charge, this dividend will represent the number of nonmetallic ions in the formula.
-6 divided by -3 = 2; therefore the other half of the formula is: Ca3N2
Example:
Potassium
phosphide
Write Symbols and the Charges:
K+1 P -3
LCM:
3
Balance the chemical
formula:
K3P

![]()
F. Binary Ionic Names:
http://www.fscj.me/Nomenclature/BinarySalts/25BinaryIonicJT.html
F. Binary Ionic Formulas:
http://www.northcampus.net/Nomenclature/BinaryIonicFormula/25BinaryIonicFormula.html
Chapter 3: Part F
Binary Ionic Compounds
Section 3.5
Using a periodic chart, write the names or the balanced formulas for the
following compounds depending on whether the formula or the name is given:
1. Copper II phosphide _________ (Cupric phosphide)
2. Iron III Oxide (rust) _________ (Ferric Oxide)
3. Lead IV sulfide _________ (Plumbic sulfide)
4. Sodium chloride _________
5. Tin II fluoride (in toothpaste) _________ (Stannous Fluoride)
6. MgCl2 ________________________
7. NiF2 ________________________
8. K3N ________________________
9. Al2O3 ________________________
10. CuBr ________________________
Chapter 3: Part E1 Polyatomic Ions Section 3.5
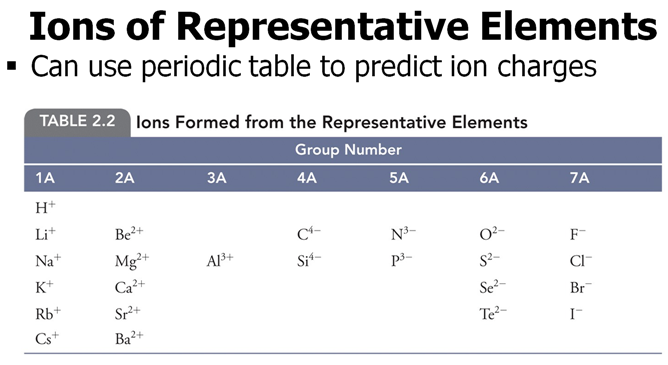
From Chapter 6 Corwin (7th) (Chapter 5 Hill), Chapter 6 Hein (14th)Monoatomic Anions or Cations can be predicted the position the element resides on the periodic chart, if the ion come from a Representative Element (IA-VIIIA) or by its name if it is a transitional metal with several different charges. Below is Corwin (7th) Figure 6.3 demonstrating common cations and anions:
Periodic Table of Selected Ions
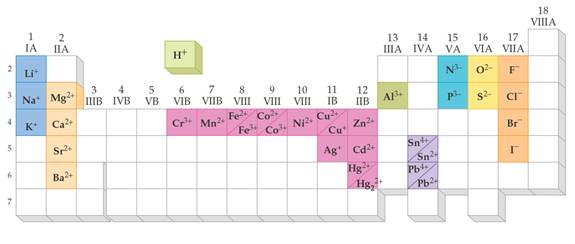
Note the charges for groups IA, IIA, IIIA, VA, VIA, VIIIA. From book to book, the charges on the transitional metals will vary
Almost all
chemistry textbooks have sections dedicated to polyatomic ions and include a
list of common ions.
What is a
polyatomic ion?
A group of atoms bound together (covalent bonds) that bears an
overall negative or positive charge.
Corwin (7th) suggests that you use flash cards listing the name on one side and the formula with its charge on the other to aide your memorization of these formulas. Most chemistry teachers require you to know some of the common polyatomic ions by the end of the course whether it is from repetition of use with a help table or from memory from the first day of introduction. Below are tables from various chemistry books used:
Polyatomic Ion Charts from Textbooks
McMurray: Table
3.2
Corwin: Table
7.03
Silverberg: Table
2.5
Tillery: Table
9.3
Kotz: Table
3.1 Hill: Table
5.04
Here is a sample polyatomic ion table:
Hill’s Table 5.4 (and
Hill suggest for you to memorize the entire table):
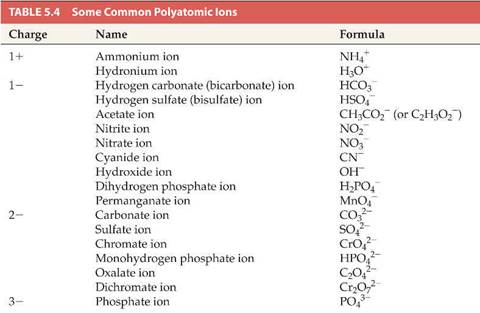
After you start memorizing, during the
course the formulas may be swimming in you head and
the charges too. To write balance Ternary Ionic Compounds, you must be able to
write the formula and the charge of each polyatomic ion required.
Corwin suggests there is only
one (Hill has two) common polyatomic Cation(s) and both end in –ium
suffix. He notes most of the Anions have an –ate suffix, while a few have –ite,
and two have –ide in
their name. How do we accomplish this list?
Knowing dot structures of
polyatomic ions (Corwin Chapter 12 section 12.5), and some keen observations
you can boil it down to six questions:
1. What is the formula
for the –ate polyatomic ion?
2. What is the charge
on –ate polyatomic ion?
3. What happens when you attach hydrogen atom(s) to the polyatomic
2- and 3- anions?
4. What does –ite
mean?
5. How do the hypo- and per- prefixes apply to polyatomic ions?
6. What are the two –ide polyatomic ions and
two -ium positive Anions?
So: it is time for you to discover, what I saw over 50 years ago. It is not in any textbook. The books just say know or memorize these tables. Go to:
http://www.fccj.us/PolyatomicIons/polyionformula.html
When you go to the site above (which
looks like the image below), click on the X for each polyatomic ion and note if
the # of oxygens is three or four in the formula.
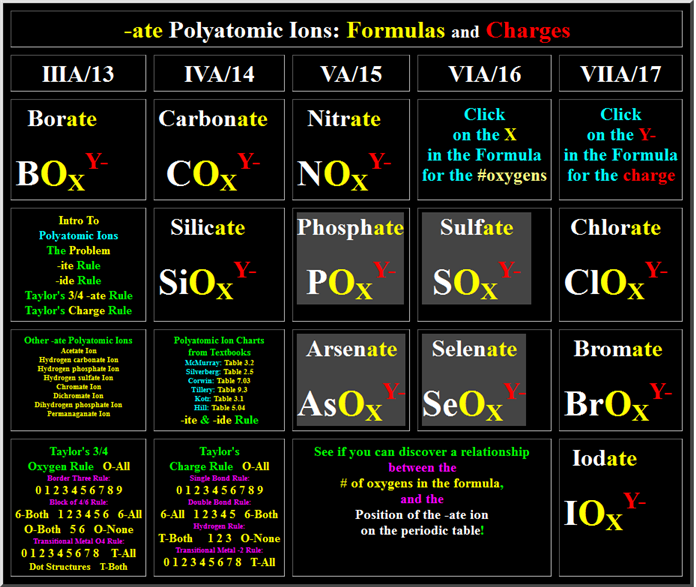
To expose the threes and the fours in the lower
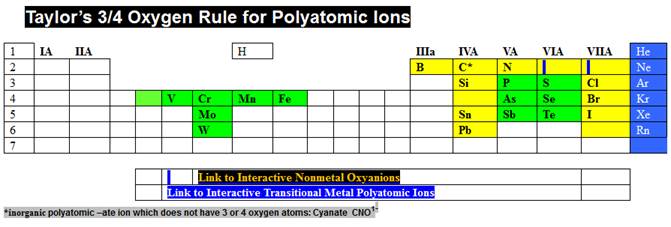
left hand corner (Taylor’s ¾ rule) click the numbers 0,1…8,9 Border three rule,
then 1,2..5,6 in the box of six rule. Also do the
0,1…7,8 Transitional O4 Rule.
Taylor’s ¾ rule is summarized at:
http://www.fccj.us/PolyatomicIons/Taylor34OxygenRuleHandout.htm
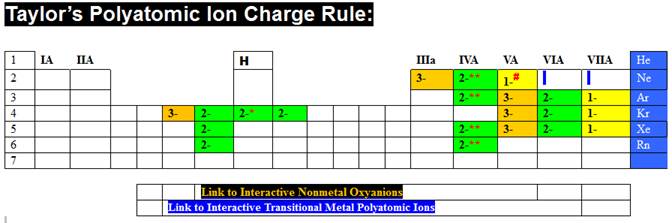
Then do the same for the box just to the right of
Taylor’s ¾ Rule, and discover Taylor’s Charge Rule.
Taylor’s Charge Rule is summarized at:
http://www.fccj.us/PolyatomicIons/TaylorChargeRuleHandout.htm
The story behind how your instructor related the
periodic table to a long list of polyions,
read the abstract for his talk at 2YC3:
http://www.fccj.us/PolyatomicIons/2YC3HowTeachPolyatomiIonsInChemistry.htm
http://www.fscj.me/PolyatomicIons/25MemorizeList.htm
E: Polyatomic Ion Names Homework: http://www.northcampus.net/Nomenclature/PolyatomicIon/25PolyatomicIon.html
E1. Polyatomic Ion Formulas: http://www.northcampus.net/Nomenclature/PolyatomicIonFormula/25PolyatomicIonFormula.html
In chemistry, a ternary compound is a compound containing three different elements. An example of this is sodium phosphate, Na3PO4. The sodium ion has a charge of 1+ and the phosphate ion has a charge of 3-. Therefore, three sodium ions are needed to balance the charge of one phosphate ion. Another example of a ternary compound is calcium carbonate . In naming and writing the formulae for ternary compounds, we follow rules that are similar to binary compounds.(CaCO3).
Site that uses least common multiple balance method:
http://web.tenafly.k12.nj.us/chemquest2/ternary_compounds.htm
Sites (You-tubes) that use the crossing method(UGH):
You-Tube: http://www.youtube.com/watch?v=8eJtYffLWKc
Another You-Tube: http://www.youtube.com/watch?v=cXyxrzUw99A
Chapter 3: Part E2 Ternary Ionic Compounds Section 3.5
Using a
periodic chart write the names or formulas of the following compounds depending
on whether the formula or name is given:
1. Na2CO3 _____________________
2. K2SO4 _____________________
3. (NH4)3PO4 _____________________
4. Ca(ClO3)2 _____________________
5. CuNO3 _____________________
6. Aluminum Hydroxide ____________
7. Ammonium carbonate ____________
8. Sodium Hypochlorite ____________
9. Magnesium Nitrate ____________
10. Iron III sulfite _____________
F: Ternary Ionic Compound Names Homework: http://www.northcampus.net/Nomenclature/TernarySalts/25ternaryIonic.html
F1. Ternary Ionic Compound Formulas: http://www.northcampus.net/Nomenclature/TernarySaltFormula/25ternaryionicformula.html
Chapter 3 Part E3
Binary/Ternary Acids (See Study Guide below)
What is an acid?
A substance that releases hydrogen ions (H+) when dissolved in water. Inorganic formulas of acids have ionizable hydrogen(s) written first in the formula.
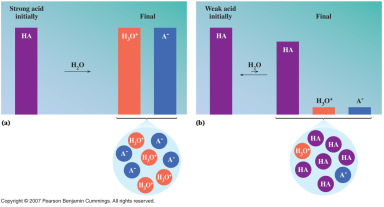
Strong Acids Weak Acids
Strong acids ionize 100% in a water solution, while Weak Acids
ionize
less than 5% in a water solution.
There are Binary/Ternary Acid online homeworks for your practice for M-4 Part G:
G: Binary/Ternary Acid Names:
http://www.northcampus.net/Nomenclature/Acids/25Acids.html
G1: Binary/Ternary Acid Names:
http://www.northcampus.net/Nomenclature/AcidFormulas/25AcidFormulas.html
( Chapter 6 Bishop
Sections 6.3-6.4 )give you instructions for naming and writing formulas of
acids. );
(Chapter 6 Corwin 7th covers
binary acids in section 6.8; while section 6.9 covers ternary acids.) (Hein 14th
covers acids in section 6.6
A brief tutorial for
names and formulas of acids follows:
If hydrogen is written first in a chemical
formula, there is two ways to name the compound. As a pure molecular compound or as an aqueous acid:
If the compound is a pure molecular compound then you name it just as if it were an ionic compound:
HCl
hydrogen chloride
HClO
hydrogen hypochlorite
HClO2
hydrogen chlorite
HClO3
hydrogen chlorate
HClO4
hydrogen perchlorate
H3PO4
hydrogen phosphate
H2CO3
hydrogen carbonate
H2SO4
hydrogen sulfate
H2SO3 hydrogen sulfite
HC2H3O2
hydrogen acetate
H2C2O4 hydrogen oxalate
HBr hydrogen bromide
HF hydrogen fluoride
Writing hydrogen
first in a chemical formula indicates that when you dissolve the compound
in water, a water molecule has the ability to pull the hydrogen off (from strong
electronegative elements like oxygen) the molecule HXO3 and
creating hydronium ions, H3O1+ and a negative ion
XO31- (cation).
The way you indicate this ionic solution is to write the formula followed by (aq) meaning a water solution: HXO3 (aq) .
The
first step is to drop the first word hydrogen and
add a second word acid:
HCl hydrogen chloride acid (aq)
HClO hydrogen hypochlorite acid (aq)
HClO2
hydrogen
chlorite acid (aq)
HClO3
hydrogen
chlorate acid (aq)
HClO4
hydrogen
perchlorate acid (aq)
H3PO4
hydrogen
phosphate acid (aq)
H2CO3
hydrogen
carbonate acid (aq)
H2SO4
hydrogen
sulfate acid (aq)
H2SO3 hydrogen sulfite acid (aq)
HC2H3O2
hydrogen
acetate acid (aq)
H2C2O4 hydrogen oxalate acid (aq)
HBr hydrogen bromide acid (aq)
HF hydrogen fluoride acid (aq)
The next step is to drop the suffix from the cation and make the following substitution with another suffix:
Change the -ate to -ic
Change the -ite to -ous
but the instead of coming up with a third suffix for -ide , they reused the -ic for -ide and added a prefix hydro- (Do not get this confused with the prefix hypo- which means 'under'.)
HCl hydrochloric acid (aq)
HClO hypochlorous acid (aq)
HClO2 chlorous acid (aq)
HClO3
chloric acid (aq)
HClO4
perchloric acid (aq)
H3PO4
phosphoric
acid
(aq) (Put the -or- syllable back in the name)
H2CO3
carbonic acid (aq)
H2SO4
sulfuric
acid
(aq) (Put the -
H2SO3 sulfurous acid
(aq) (Put the -
HC2H3O2
acetic acid (aq)
(Notice
the three hydrogens written after carbon are NOT ionizable and not written
first in the formula)
H2C2O4 oxalic acid (aq)
HBr hydrobromic acid (aq)
HF hydrofluoric acid (aq)
On Corwin 7th
page 185 Questions 49-56 will give you more practice on writing names and
formulas of acids.
At the end of
chapter 6 Hein 14th exercises 17, 18, 19, and 20 pages 116-117 are
additional acid nomenclature problems.
Chapter 3 Part E3 Binary/Ternary Acids
Using a
periodic chart write the names or formulas of the following compounds depending
on whether the formula or name is given:
1. HCl _____________________
2. H2SO4 ____________________
3. HNO3 _____________________
4. HNO2 ___________________
5. H2CO3 ___________________
6. Hypochlorous acid _________
7. Phosphoric acid _________
8. Sulfurous acid _________
9. Perchloric acid _________
10. Hydrofluoric acid ________
E3:
Binary/Ternary Acid Names Homework: http://www.northcampus.net/Nomenclature/Acids/25Acids.html
E3.1.
Binary/Ternary Acid Formulas:
http://www.northcampus.net/Nomenclature/AcidFormulas/25AcidFormulas.html
Submit
grades on separate grading Sheet when taking M-4 Exam
Online Study
Guide:
http://www.fccj.us/chm1025/AssignmentOutline/M4PartG.htm
Chapter
3: Part E4 Inorganic Compounds
The key to deciding which system to use in Part H is to look at the element written first.
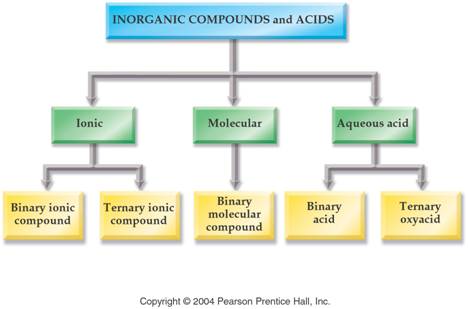
1. If a Metal is written first (or a polyatomic ion), then use the rules for ionic compounds (salts).
2. If a nonmetal is written first, then use the Covalent/Molecule System with prefixes. (If the compound is Organic Nomenclature of Organics is covered in Chapter 11, but for now use the prefix system of binary molecular nomenclature.
3. If hydrogen is written first (and it is in aqueous
solution) then name it as an Acid
Chapter 3:
Part G Matter Chart Sample Test Section
3.7
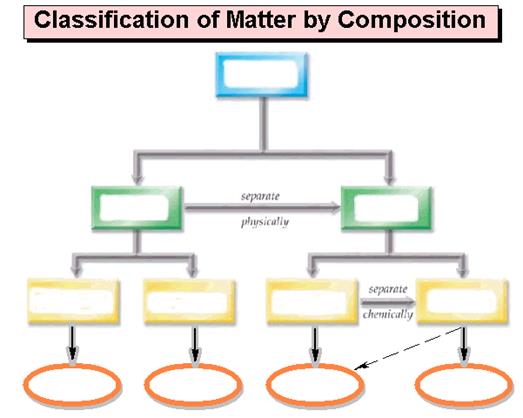
Draw below a matter chart similar to the chart in section
3.2 page 68 of the Corwin
Introductory Chemistry text,, Figure 3.28 page 78
Suchocki text, or it may be of your own design as long as it clearly denotes
lines which describe which words are subunits of the more general word. The chart should include the following: homogeneous mixtures, heterogeneous mixtures,
Matter, Pure Substances, Mixtures, Compounds, Elements, Solutions, Atoms,
Molecules/Formula Units, and Colloids/Suspensions. Also draw/label the arrows: Separate Physically and Separate Chemically:
Fill in Below
Matter Chart Homework Critical Thinking:
Chapter 3: Part G1 Matter Chart-Critical
Thinking Application
1..Where would you place: colloids in the matter
chart?
2. If you subdivided Inorganic Compounds and
Organic Compounds under compounds., sketch below where would you put:
Salts, Acids, Bases, Covalent Compounds?
3. Sketch below and show under which
subdivision would you put:
Electrons, Protons, and Neutrons
4. . Sketch below and show under which subdivision
would you put:
electrons, protons, neutrons, nucleus, orbitals
Fill in Below:
Chapter 3
Part P Periodic Properties
Section 3.3


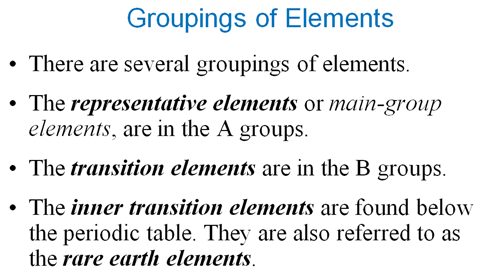
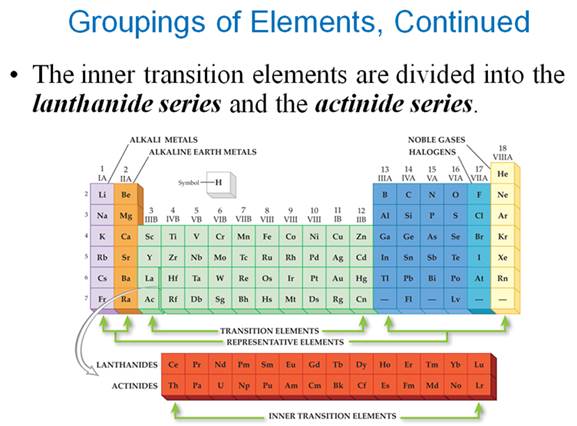
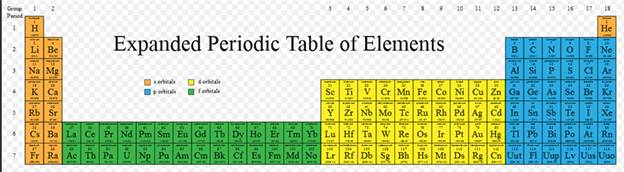
Chapter 3 Part P: Periodic Chart
Identification
Selected symbols
have been placed into the following blank periodic table of elements:
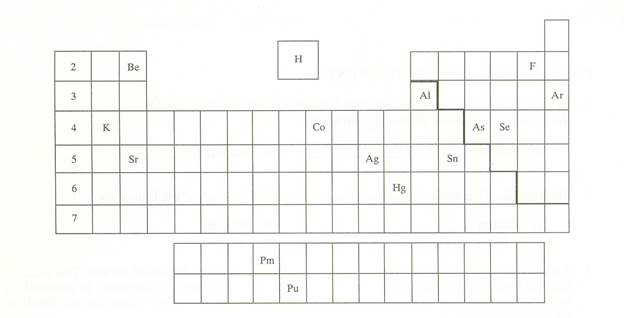
Which symbol in the above periodic table
fits the following description?
_____1. an alkali metal
_____2. A halogen
_____3. an alkaline earth element
_____4. a noble gas
_____5. A representative element in the
fifth period
_____6. a semimetal
_____7. An element in the lanthanide series
_____8.
an element with the atomic number 13
_____9. an element filling a 5d sublevel
_____10. an element with six valence
electrons
_____11. an element corresponding to: 1s2
2s2 2p6 3s2 3p6 4s2 3d7
_____12. an element with four valence
electrons
_____13. an element in the actinide series
_____14. the main isotope of this element
has zero neutrons in the nucleus
_____15. a representative element in the
first period of the periodic table
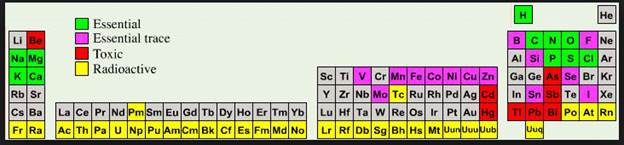
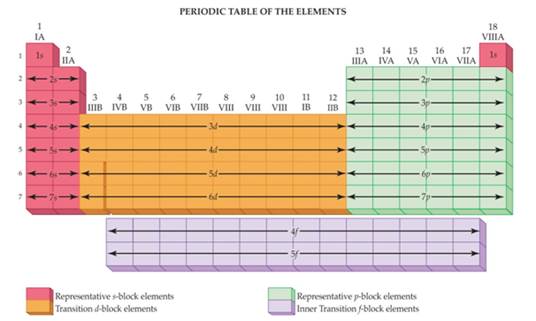
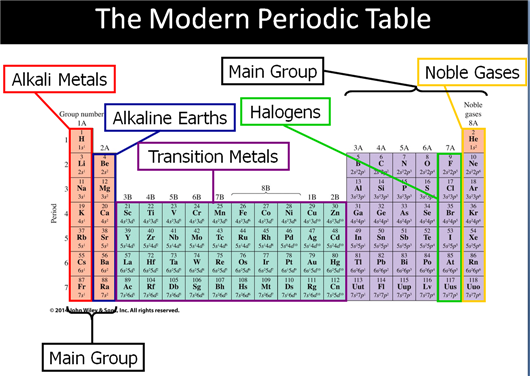
Main Group Elements are also called Representative
Elements





Periodic table's
seventh row finally filled as four new elements are added
Discovery of four super-heavy chemical elements
by scientists in Russia, America and Japan has been verified by experts and
formally added to table
Four new elements have been added to the
periodic table, finally completing the table’s seventh row and rendering
science textbooks around the world instantly out of date.
The elements, discovered by scientists in
Japan, Russia and
America, are the first to be added to the table since 2011, when elements 114
and 116 were added.
The four were verified on 30 December by the
US-based International Union of Pure and Applied Chemistry, the
global organization that governs chemical nomenclature, terminology and
measurement.
IUPAC announced that a Russian-American team of scientists at
the Joint Institute for Nuclear Research in Dubna and
Lawrence Livermore National Laboratory in California had produced sufficient
evidence to claim the discovery of elements 115, 117 and 118.
Period drama: the story
of the periodic table
The body awarded credit for the discovery of
element 113, which had also been claimed by the Russians and Americans, to a
team of scientists from the Riken institute in Japan.
Kosuke Morita, who was leading the research at Riken,
said his team now planned to “look to the unchartered territory of element 119
and beyond.”
Ryoji Noyori, former Riken
president and Nobel laureate in chemistry said: “To scientists, this is of
greater value than an Olympic gold medal”.
The elements, which currently bear placeholder
names, will be officially named by the teams that discovered them in the coming
months. Element 113 will be the first element to be named in Asia.
“The chemistry community is eager to see its
most cherished table finally being completed down to the seventh row,” said
Professor Jan Reedijk, president of the Inorganic
Chemistry Division of IUPAC.
“IUPAC
has now initiated the process of formalising names
and symbols for these elements temporarily named as ununtrium, (Uut or element 113), ununpentium (Uup,
element 115), ununseptium (Uus,
element 117), and ununoctium (Uuo, element 118).”
Since the 19th century, European and American discoveries have monopolized the naming of elements on the periodic table. It is evident in entries like francium, germanium, scandium, polonium, europium, californium, berkelium and americium.
But now, for the first time, researchers in Asia will make an addition to chemistry’s most fundamental catalog.
Scientists from the Riken institute in Japan will bestow an official name on Element 113, currently known by the placeholder name ununtrium, the International Union of Pure and Applied Chemistry announced last week.
The organization said that studies published by the Japanese scientists from 2004 to 2012 give the team the strongest claim to having discovered the element. The declaration comes more than 12 years after the Japanese team first attempted to synthesize the superheavy element, by firing beams of zinc at a thin bismuth film.
Led by Kosuke Morita, the group began to bombard bismuth atoms in a particle accelerator at 10 percent the speed of light in 2003. A year later, they successfully fused two atomic nuclei from these elements, creating their first nucleus of Element 113, but it decayed in less than a thousandth of a second. In 2005, the team produced Element 113 in a second event, but the chemistry union did not consider the demonstration strong enough to denote a discovery.
What Would You Name a New Element?
Imagine that you could name a new element on the periodic table. Send your ideas to scitimes@nytimes.com with a 50-100 word explanation. Before your imagination gets away, consider these published guidelines for new elements (For linguistic consistency, the names of all new elements should end in “-ium”). In keeping with tradition, elements are named after:
- A mythological concept or character (including an astronomical object);
- A mineral, or similar substance;
- A place or geographical region;
- A property of the element; or
- A scientist.
“For over seven years, we continued to search for data conclusively identifying Element 113, but we just never saw another event,” Dr. Morita said in a statement. “I was not prepared to give up, however, as I believed that one day, if we persevered, luck would fall upon us again.”
In 2012, the team finally produced strong evidence that they had synthesized Element 113. Over the course of those nine years, the beam was active for 553 days and launched more than 130 quintillion zinc atoms, according to Nature.
The chemistry union, along with the International Union of Pure and Applied Physics, granted the Riken researchers naming rights to Element 113 over a joint Russia-United States team that had also claimed to discover the element.
The chemistry union’s decisions are detailed in two reports to appear in the journal Pure and Applied Chemistry. In addition to Element 113, Elements 115, 117 and 118 will also receive official names. Teams from Russia and the United States discovered those elements.
With their discovery, the bottom row of the periodic table will be complete. Elements are numbered by the protons they have in their nucleus, and Elements 114 (flerovium) and 116 (livermorium) had previously been confirmed and named.
Dr. Morita has not yet announced what he intends to name Element 113, but according to a 2004 article in The Japan Times when the team first published its results, one likely contender may be “japonium.”