CHM
1025C Module 2
Homework Packet Name:________________________
Module 2i: ChemMath and Measurement (Hein 14th Chapter 2)
A. _____(02) Significant Figures- Section 2.2 Answers
B. _____(02) Round Off/Math of Significant Figures- Section 2.3/2.4 Answers
C. _____(02) Scientific Notation Section 2.1 Answers
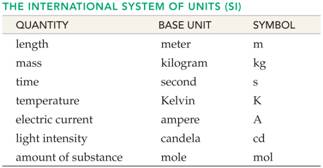
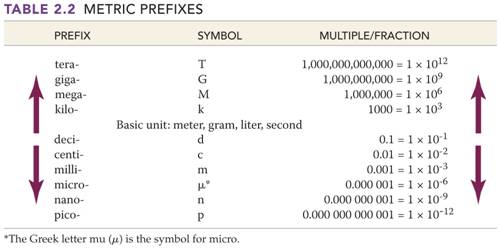
D. _____(03) Metric Basic Units /Numerical Prefixes- Section 2.5Table 2.1 Answers
E. _____(03) Metric System Conversion Factors- Section 2.5Answers
_______(12)
Module 2i Total (Third Exam)
Module 2ii: ChemMath and
Measurement (Hein 14th Chapter 2)
F. _____(09) Unit Analysis Sections 2.6 Answers Pretest #2 Ans2 Online Site
G. _____(03) Temperature Conversion Section 2.7 Answers
H.
_____(02) Density/Specific
Gravity/Volume Calculations Section 2.8 Answers hi
I. _____ (02) Specific Heat Calculation/Ice Cube Problem Section 4.5 Answers hi
_______(16)
Module 2ii Total (Fourth Exam)
Module Two: Homework Packet
Module Two-Part A: Significant figures 2 points
In the
blank, state the number of significant figures in each of the following
measurements:
____1. 0.05 mL
____2. 250.0 cm
____3. 456,000,000 people
_____4. 1000 g
_____5. 0.00006500 moles
_____6.
0.00200 kg
_____7.
50 seconds
_____8.
50.0 Seconds
_____9.
50.00 Seconds
_____10.
0.05 Seconds
Significant Digit Animation:
http://www.lsua.info/chem1001/Chap2-3Movies/sigdigit.html
Module 2 Pretest Homework Packet
Module Two-Part B: Rounding Off & Arithmetic
Operations of Sign. Figures 2 points
Round
off the following numbers to three significant figures:
(1)
1.598 x 106 = _____________
(2)
0.000 000 484 500 = _________________
(3) 0.01045 =
_______________
(4) 1.98754 X10-7 = ________________
Perform the following addition/subtraction/multiplication/division
operations and express the answer using the proper units and significant
figures:
(5) 4
mL
16.3
mL
+ 0.953
mL
(6) 376.5 mL
- 76
mL
(7)
16.5 cm
X 1.7 cm
(8) 12.0 g ÷ 1.00 g =
or
12.0 g
/ 1.00 g =
(9)
9.2 cm X
9.20 cm
X 3.14 X 22.65cm
=
(10)
(5398 cm3 – 2060.2 cm3) /16.8 cm3/sphere =
Module 2 Pretest Homework Packet
Module Two-Part C: Exponential Numbers and Scientific
Notation 2 points
Express
the following ordinary numbers in scientific notation (If greater than three
significant figures, round off to three significant figures:
(1) 1,010,100,000,000, 000 = ________________
(2) 0.000 000 000 000 019 = ________________
(3) 456,789 = _________________
(4)
0.0001198 = _____________
(5)
1,000,000 = ______________
(6) 0.000200 = ______________
(7)
Express the following
products in exponential form
2 X 2
X 2 X 2 X 2 X 2 X 2 X 2 = ______________
(8)
and use your calculator to calculate the value:
Value
= ___________________
(9) Express the following powers often notation:
1 x 100
= ______ 1 X 101=______ 1 x 10-1 = _________
(10)
Express the ordinary number in scientific notation in three significant
figures:
60,230,000,000,000,000,000,000
= _______________________
Module 2 Pretest Homework Packet
Part D: Metric
System Basic Units/Numerical Prefixes
3 points
Fill in the blank with the proper basic
unit or metric prefix, then in the parenthesis put the unit’s or prefix’s
abbreviation (Use table from Chapter 3):
____________( )
1. Basic unit of length in the metric system
____________( )
2. Basic unit of volume in the metric system
____________( )
3. Basic unit of mass in the
metric system (not SI)
__________( )
4. Metric prefix which means 1/1000 of a unit
__________( )
5. Metric prefix which means 1000 units
__________( )
6. Metric prefix which means 1/100 of a unit
__________( )
7. Metric prefix which means 1/10
of a unit
__________( )
8. Metric prefix which means 1,000,000
units
__________( )
9. Metric prefix which means
1/1000000 ( 10-6)
of a unit
__________( ) 10.
Metric Prefix which means 1/1000000000 (
10-9) of a unit
Metric Prefix Table:
http://www.lsua.info/MetricSystem/MetricPrefix.html
Metric System Animation:
http://www.lsua.info/chem1001/Chap2-3Movies/metric.html
Module 2 Pretest Homework Packet
Module Two-Part E
Metric Unit Factors 3 points
Fill in the blank with the number which
completes the metric unit factor:
(1)
__________mg = 1.000 g
(2)
__________mg = 1.000 kg
(3)
__________mL = 1.000 L
(4)
__________cm = 1.000 m
(5)
___________mL = 1.00 cm3
(6)
____________km = 1.000 m
(7) ____________ g =
1 kg
(8) ____________ cm = 1 dm
(9) ___________ µL
= 1 L
(10) __________ nm = 1 m
(11)
Write a unit equation for each of the following metric equivalents:
(a) M and Tm (b) L and mL (c) Bytes and G-bytes
(a) ______________ (b) ____________ (c)
________________
|
|
|
Module 2 Pretest Homework Packet
Part F: Unit
Analysis Problems 9 points
(Work any 9-you must show dimensional
analysis sequence for credit)
Apply the unit analysis method of
problem solving to each of the following
( If greater than three significant figures round off to three
significant figures):
Problem 1
An
oxygen molecule travels 975 mi/hr at room temperature. There are 5280 ft = 1
mi; 12 in = 1 ft, 2.54 cm = 1 in, 1.6 km = 1 mi, and 3600 sec = 1 hr. What is
the velocity in meters per second?
Problem 2
If one gram is equal to 15.4 grains. How many 5.00 grain
aspirin tablets may be made from 1.00 kilogram of aspirin?
Problem 3
A
parsec is the distance light travels in 3.26 years. Given the velocity of
light, 3.00 x 108 m/sec, how many kilometers does light travel in
one parsec?
Problem 4
I have
1400 radio programs I want to put on an Apple Ipod. Each program requires 5
megabytes of disk space. If there are 1024 megabytes in a
gigabyte. How many gigabytes of disk space do I need minimum to store
all my programs on the IPod. The Mini-Ipod holds only 4 gigabytes of recordings, could I use a mini for my project?
Problem5
Find the mass in grains of a 325 milligram aspirin tablet.
(Given: 1.00 g = 15.4 grains)
Problem6
Insurance statistics state that a person loses 8 minutes of average life for
each cigarette smoked. If there are 20 cigarettes in a pack and the average
cost of cigarette is $5.00 per pack over the next 25 years, how many years of
average life would a person lose for smoking 1.5 packs a day for 25 years?
Problem7
What is the density of water in lb/ft3, if the density of water at
25oC is 1.00 g/ml?
[Hint: There are 2.54 cm = 1 in (or 16.48 cm3
= 1 in3); 454 g = 1 lb ]
Problem8
Calculate the velocity of a car traveling car traveling 65 miles/hr in ft/sec.
Problem9
How many milligrams does a 0.750 carat diamond weigh?
(Hint: 1 carat = 0.200 g)
Problem10
Diamond has a density of 3.513 g/cm3. The mass of a diamond is often
measured in carats, 1 carat equaling 0.200 g. What is the volume of a 1.50
carat diamond?
Problem11
Liquor used to be sold in fifths. A fifth is one fifth of a gallon. A gallon is
128 fluid ounces. Today liquor is sold in bottle sizes of 750 ml to equate to
the old fifth. If there are 946 ml in a quart, calculate the number of
milliliters in a fifth. How many milliliters difference is there in the
bottling?
Additional Homework (not required) for your practice:
Corwin 7th edition: Page 59: #19 - #26
Hein 14th edition: Page 40-41: #23- #42
Problem 12
1. On
July 23, 1983 Air Canada Flight 143, flying at 26,000 feet from Montreal to
Edmonton, ran out of fuel because the first officer ask
the mechanic for the conversion factor of mass to volume at Montreal. The
mechanic gave the first officer the answer 1.77 with no units. The plane had
7682 L of fuel at Montreal. The pilot knew he needed 22,300 kg of fuel to make
the trip. The mechanic's answer of 1.77 was pounds per liter not kilograms per
liter caused the error such that only 4917 L of fuel was added. If there are
2.205 pounds in a kilogram, how many liters of fuel were needed for the trip?
How many liters minimum of fuel should have been added at Montreal before
takeoff?
Problem 13
Before
1982 the US Mint cast penny coins from an alloy of copper and zinc. A 1980
Penny weighs 3.051 g and contains 2.898 g of pure copper. In 1982 the US Mint
stopped making copper pennies, because the price of copper was worth more than
the penny. The post 1982 penny contains only a layer of copper over zinc. A
1990 penny weighs 2.554 g and contains 2.490 g of zinc. If the mint melted down
one pound of 1980 pennies, how many 1990 pennies can be made from the total
copper from the 1980 pennies?
Problems 14
An
Olympic size swimming pool is 50.0 m long and 25.0 m wide. How many gallons of
water ( d = 1.0g/mL )are needed to fill the pool to an
average depth of 5.5 feet.
Problem 15
A
furniture factory needs 29.5 ft2 of fabric to upholster one chair. A
Europen supplier sends the fabric in bolts of exactly 200 m2. What
is the maximum number of chairs that can be upholstered by three bolts of fabric. Hint: 1 m - 3.281 ft)?
Problem 16
My throw
away car gets 23.4 mi/gal and hold 70.1 L of gasoline. How far can I drive on a
tankful of gas?
If gas
cost $3.49/gal; how much does a tankful of gas cost?
If the
average speed on a trip is 92.2 km/hr, How many hours
may I drive the car on the trip before I run out of gas?
Module 2 Pretest Homework Packet
Part G Temperature
Conversion 3 points
The general formula
for the conversion of temperatures on scale X to temperatures on scale Y is:
oY = Y units/ X units ( oX - RPx) + RPy
1.Write
the formula for the conversion of Fahrenheit to Celsius:
2. Write the formula for the conversion
of Kelvin to Celsius:
3. convert
-196oC to oF
4. convert
-196oC to K
5. The Rankin scale uses a Fahrenheit
unit, but assumes zero to be absolute zero.
If absolute zero on the Kelvin scale is zero and on Celsius scale is
-273oC, calculate
absolute zero on the Fahrenheit scale, then estimate the Freezing point of
water on the Rankin scale. (The BP water=212
oF=100 oC=373K)
Additional Homework (not required) for your practice:
Corwin 7th edition: Page 60: #59 - #66
Hein 14th edition: Page 41: #43 - #50
Module 2 Pretest Homework Packet
Module Two-Part H: Density, Specific Gravity & Volume
Problems 2
points
1. A
quartz rock was cut into a rectangular solid paperweight. IF the paperweight has a mass of 165 g and
measures 5.00 cm by 5.00 cm by 25.0 mm, what is its volume in cubic
centimeters?
2. Calculate the density in g/mL for 10.0 grams of ethyl ether having a volume
14.0 mL.
Additional Homework (not required) for your practice:
Corwin 7th edition: Page 60: #43-#46; #47 - #58
Hein 14th edition: Page 41: #51-56
Part I: Heat and
Specific Heat 2 points
- Find the specific heat of gold if 25.0 cal is required to heat
30.0 g of gold from 27.7 oC to 54.9 oC.
- Calculate the heat required to raise to raise 25.0 g of iron (sp
Heat=0.108 cal/g ∙ oC) from 25.0 oC to 50.0 oC.
Additional Homework (not required) for your practice:
Hein 14th edition: Page 77: #24-34